Tripropylene Glycol Diacrylate (TPGDA) came onto the industrial scene with a bigger push in the latter part of the twentieth century. Chemical companies turned to it as a way to meet the growing need for rapid-curing coatings and inks in manufacturing. Early formulations of acrylates, dating to the post-war era, struggled with issues like brittleness or low reactivity. TPGDA landed as a fix, offering a sweet spot between workability and physical durability. Paint factories and printing shops latched on quick. Using it, operations cut down drying times without losing scratch resistance. My own experience in a resin blending shop in the late 1990s drives home how quickly this material could become part of the workflow—not only for specialty applications, but for everyday floor coatings, flexible printing plates, and even formulations for dental materials. Growth hasn’t stopped; the market for TPGDA keeps getting fatter, with newer uses popping up beyond the standard UV-curable inks or adhesives.
TPGDA works as a reactive diluent, blending into many acrylate systems to reduce their thickness and help the resin flow better. Liquid at room temperature, it pours off the drum with a faint smell. Chemically, it carries two acrylate groups stuck onto a tripropylene glycol backbone, allowing for quick reactions under UV light or electron beams. Factories choose TPGDA for both performance and cost; it competes well against monofunctional or trifunctional acrylate cousins in cost per kilo, giving formulators flexibility without big trade-offs in cure speed or mechanical properties. Beyond the chemistry jargon, using TPGDA means you can churn out coatings that dry in seconds, hold up under wear, and still retain some give—never too brittle, never gummy.
TPGDA rolls out clear and almost colorless. Viscosity drops into a sweet range—neither runny as water nor thick as honey, hovering around 100 mPa·s at room temperature. It mixes easy with other acrylates, pointing to good compatibility. Specific gravity sits a bit over 1, so one liter weighs a little more than a kilo. Flash point checks in higher than many solvents, reducing fire risk. TPGDA resists yellowing in the final product and boasts low volatility, making it safer for factory workers. Its diacrylate structure lets it link together into long, tough chains at lower energies than some older resins—a key for curing under energy-efficient lamps. An extra hydroxyl here and there adds some flexibility, which helps absorb shock in the finished cured polymer.
Industry standards call for tight controls on TPGDA’s purity and residuals. The major producers publish technical data sheets listing minimum content of the diacrylate, max levels of residual monomers, water content, and stabilizer types. Purity typically clocks in at 98% or above. Labels warn users of sensitization hazards and recommend gloves and goggles. Transport follows UN rules for flammable liquids but earns a lower risk score than some older monomers. MSDS sheets always advise plenty of ventilation, especially in sunlight-exposed warehouses. The bottle or drum almost always states the batch number, production date, and lists the weight by net content, not just volume, given the slight difference from water. In the EU, TPGDA falls under REACH registration, so producers share longer lists of impurities, down to the ppm.
Manufacturers synthesize TPGDA through a straightforward but tightly controlled process. It starts with tripropylene glycol, a by-product-heavy cousin of propylene glycol, and this base reacts with acrylic acid, driven along by acids or metal salt catalysts. Excess acrylic acid gets stripped away in the wash. The process produces little waste if careful attention goes toward keeping reactor temperatures steady. Even small slips in heat can trigger runaway reactions or unwanted byproducts like diacrylic acid, throwing whole shipments out of spec. Large plants recycle leftover monomer, filtering and purifying before looping back into the reactor, squeezing every bit of value from raw material costs.
Once made, TPGDA steps into reactive formulations, linking up through free radical polymerization. This means, once exposed to initiators like benzoin ethers under UV light or peroxides in thermal curing, it instantly connects its acrylate ends to form tough polymer chains. Because of its two reactive sites, it forms cross-links instead of straight spaghetti strands—so the finished resin resists dissolving and holds up to stress. Chemists sometimes tweak TPGDA by partial esterification, blending different glycol backbones or changing the acrylate ends to tune speed or mechanical strength. The industry also experiments with adding functional side groups, letting the final networks grab dyes, bind metals, or even host antimicrobial agents.
On paper and in shipping manifests, TPGDA shows up under nearly a dozen names. Most common labels you’ll spot are: Tripropylene Glycol Diacrylate, TPGDA, and sometimes by trade names tied to big chemical suppliers—like Sartomer’s SR306 or BASF’s Laromer TPGDA. International markets use product codes or adjust spelling for local rules, but the core chemical structure always matches. Always check the molecular formula if you’re swapping vendors; differences in purity or stabilizer content slip in under the same trade name, depending on the region or batch origin.
From my own days handling TPGDA, it was the potential for skin sensitization that grabbed everyone’s attention—not fumes or fire. Wearing gloves and long sleeves stopped irritation in most cases. Spills on concrete cured to a hard puck in sunlight, and ventilation kept vapor below exposure limits even in big tank rooms. Safety regulators require closed-batch transfer, splash guards at filling stations, and regular skin checks for repeated users. European oversight includes workplace exposure limits near 1 mg/m³ as an 8-hour TWA. US guidelines from OSHA urge training sessions on spill handling and personal protective equipment, with first-aid washing stations in reach. There’s always a warning about mixing leftover acrylates or letting anything pool near hot equipment, since polymerization kicks off with enough heat or light. Facilities store TPGDA in double-wall tanks, often blanketed with inert gas to block stray curing.
Shops and plants pull TPGDA into dozens of end products. In the print industry, it unlocks fast-setting inks and varnishes—labels look glossy and stand up to fingerprints. Electronics makers use it in solder mask coatings, where tight line definition and no yellowing matter most. The coating world benefits the most, rolling out floor finishes that withstand forklifts and UV-cured wood stains that stay scratch-free. Dental labs switch to TPGDA for accurate crowns and bridge molds, where low shrinkage matters. Lately, 3D printing circles add it to custom resin blends, balancing flow and toughness—letting hobbyists and engineers print both delicate models and strong, articulated joints. Medical devices rely on TPGDA’s predictability; manufacturers secure reliable tube and chamber coatings. Where a quick cure and solid performance count, TPGDA often lands on the short list of raw ingredients.
Labs keep hunting for new ways to leverage TPGDA’s backbone. Academic groups blend it with bio-based acrylates, working to cut the environmental punch of petroleum origins. A few startup labs experiment with adding nanoparticles to the mix, aiming for scratch-resistant, antibacterial coatings for hospitals. Others hunt for even faster initiators, cranking out coatings under weaker lamps and investing less in power. Big players chase new end-uses—collaborating with 3D printer manufacturers, or developing TPGDA-based adhesives that grip car trim but pull clean during repairs. The drive for “greener” profiles pushes chemical engineers to lower the toxicity of initiators and stabilizers mixed into TPGDA-based systems without losing the rapid, strong cure that customers expect.
Toxicologists began ringing bells about acrylates in the ‘80s, after factory workers developed skin rashes and minor respiratory irritations. Long-term studies on TPGDA prove it doesn’t cause cancer, but skin sensitization still raises concern, leading formulators to cap the content or add less reactive co-monomers. Inhalation studies track little systemic toxicity at measured workplace levels, especially in ventilated settings. The acrylate’s reactivity means it rarely persists in the body or environment, reducing bioaccumulation risk. Regulatory agencies check any chronic overexposure risks—especially for at-risk workers or those recycling cured scrap. Waste handling keeps cured solids separate from liquid waste streams, keeping waterways free of unreacted monomers. Modern MSDS sheets warn workers about hand-to-mouth contact and advise robust washing after handling, keeping accidental ingestion risks almost nil.
Looking forward, TPGDA seems well-placed to hold its ground. Manufacturing trends point toward more digital, on-demand printing and precision layering—both sweet spots for TPGDA chemistry. With sustainability at the front of conversations, some chemists eye routes to make the glycol base from renewable feedstocks rather than petrochemicals. In packaging, the push for recyclable plastics spotlights UV-cured coatings that keep food safe with less or no solvent emissions. As I’ve seen in project meetings, young engineers ask for drop-in replacements that keep the workhorse features—speed, wear resistance—but also slice carbon impact. That challenge presses suppliers and researchers to stretch TPGDA, either with greener catalysts, recycled inputs, or radical new end-uses that blend performance with responsibility. As regulatory targets tighten, TPGDA’s track record of reliability and adaptability may help it steer through both market and legal shakeups.
Walk into any electronics store, pick up a glossy magazine, examine a new smartphone, or stroll down the hardware aisle. The shine, the scratch-resistance, the fine print—behind all these modern finishes, you’ll often find chemicals pulling the weight. One of those unsung heroes is tripropylene glycol diacrylate, or TPGDA as the folks in the industry call it. At first glance, its name sounds niche, but the reach goes far.
TPGDA shows up most obviously in the world of inks and coatings. Digital printing, especially with UV inks, wouldn’t get the clarity or durability it does without TPGDA. Every time you run your finger across a color-rich print that resists smudging, that’s this compound working in the background, cross-linking with other acrylates under UV light to lock colors in place. It’s not just about artistry or marketing either—this helps extend shelf life and cut down on waste.
You’ll also see TPGDA wherever there’s a need for a tough surface without the hassle of traditional drying times. Shop floors, garage workshops, and hospitals all rely on fast-curing floor coatings. TPGDA helps these coatings set up quickly under ultraviolet lamps, so spaces reopen in hours instead of days. I remember redoing my garage floor—the installer talked about using a UV-cured resin containing TPGDA because it meant not having to clear out space for a week. Fast turnaround means less disruption to daily life, which everyone can appreciate.
Sleek phone screens owe some of their scratch resilience to the tough, thin layers formed by acrylates like TPGDA. Printed circuit boards—the beating heart of any modern device—must weather heat and moisture, often in tight spaces. TPGDA helps engineers hit those marks by delivering polymer layers that go on fast and hold up well under pressure. With demand for smaller, more powerful devices growing each year, manufacturers turn to TPGDA’s balance of flexibility and strength to hold components in place and fend off day-to-day wear.
Dentists and orthodontists have also adopted TPGDA-based composites for quick-setting fillings and dental appliances. Cure times plummet because the acrylate turns solid under UV light. In dental offices, time equals pain avoided and cost saved. Factories running 3D printers often use acrylate-based resins when features must be sharp and detail matters. TPGDA flows easily, then locks down under UV cure, shaping everything from industrial prototypes to custom medical implants.
Just because TPGDA does its job well doesn’t mean it’s problem-free. Overexposure before the compound sets can cause skin irritation or allergic reactions for some workers. Keeping a safe workshop means gloves, proper handling training, and well-ventilated spaces. From what I’ve seen in both large plants and small craft shops, those precautions make all the difference. Industry leaders and researchers keep working on safer alternatives and better personal protective gear, pushing safety to the top of the list.
While some companies experiment with alternative resins and greener acrylates, TPGDA sets a high bar. Its unique blend of durability, fast cure, and versatility will keep it relevant as long as consumer expectations for quality and performance grow. Responsible handling, honest labeling, and ongoing research into safer chemistries shape the factory of tomorrow.
Walk through any printing shop or factory making modern plastics and you’ll often find tripropylene glycol diacrylate behind the scenes. This clear, viscous liquid gives coatings durability and helps inks set fast under UV light. Its popularity in adhesives, paints, and electronics has grown thanks to industries chasing speedy production and tough results. Still, health questions keep coming up, especially for the people who mix, pour, and cure resins with their own hands. Does contact with this chemical mean real risk?
Spend years in a facility where these resins move from drum to machine, and it’s pretty clear that chemical exposure isn’t always about spills or major incidents. Much of the time, it comes down to splashes, vapors, or tiny droplets on sleeves. Those who handle tripropylene glycol diacrylate regularly tell stories about skin irritation and rashes. Some itching or mild redness might seem minor at first, but repeated exposure sharpens the risk of more intense reactions. Scientific studies have linked acrylate compounds to allergic contact dermatitis. European regulatory agencies have underscored the importance of personal protection, like gloves and goggles, as direct touch over weeks or months turns problems from minor irritation into painful, persistent conditions.
Heat a vat, run a fast production line, and small amounts of vapor head straight into the air. Tripropylene glycol diacrylate isn’t especially volatile at room temperature, but warm the room by a few degrees and people nearby might start to smell something faintly sweet or chemical. Respiratory irritation pops up in cases where ventilation falls behind. Breathing in fine aerosolized particles can provoke coughing, throat discomfort, or headaches. Health surveys among factory technicians around the world echo these symptoms, especially when facilities skip local exhaust ventilation.
Dig deeper, and the bigger worry circles back to sensitization. Some folks might handle the compound for months without trouble, only to develop persistent allergies later on. There’s no strong link to cancer after routine workplace exposure, according to institutions like OSHA and IARC. Still, the allergic reactions—especially for hands, forearms, and eyes—can push skilled workers to different jobs or force early retirement. For most members of the public, there’s almost no direct risk, but those making and applying these products come first in harm’s way.
Tighter workplace controls can drop the rate of skin problems and asthma-like symptoms. Simple gear—chemical-resistant gloves, washable aprons, proper eyewear—works wonders. Regular training and safety briefings keep everyone alert to potential risks, especially for new hires and late-shift staff. Automated mixing and closed transfer systems cut the chances of accidental spills or vapor leaks. Every safety manager I’ve spoken with stresses ventilation: fans over open vats, vented enclosures around curing stations, and scheduled checks for air flow.
Finished products—those shiny phone cases, glassy coatings, and printed electronics—almost always trap the chemical inside a tough polymer, so everyday contact isn’t much of a hazard after curing. Still, everyone involved in the early stages of manufacture deserves clear warnings, strong safety cultures, and a work environment that keeps the invisible risks in check.
People who handle chemicals like Tripropylene Glycol Diacrylate (TPGDA) quickly learn that safety and quality come down to details. On a shop floor or in a warehouse, real-life risks and cost matter far more than textbook rules. TPGDA, a colorless liquid used in inks, adhesives, and coatings, calls for a sharp eye and careful setup. If a drum ends up in the wrong spot or exposed to the wrong conditions, a small misstep snowballs into loss, downtime, or even harm to workers.
TPGDA holds up best in a climate-controlled place. Even folks with decades in chemical manufacturing agree that stable, moderate temperatures stop a world of headaches. Heat and sunlight speed up polymerization. The liquid thickens, gels, or sets off by itself, and no one wants to deal with hardened resin plugging lines or ruining a batch. The ideal spot stays below 30°C (86°F) and never freezes. A plain metal shed with hot metal walls messes with shelf life, but a shaded, insulated room avoids drama.
Ventilation proves crucial, especially during hot spells or when a spill occurs. TPGDA can release low levels of vapors, which hang around in still air. Anyone who’s spent time in musty storage rooms knows that air movement avoids headaches, both literal and legal. Good airflow flushes out vapors, keeping levels below occupational exposure limits. It also cuts down on the chances of buildup, which matters most if a drum gets knocked over and the smell starts creeping through the building.
Some people overlook how big a role light plays with acrylates. TPGDA reacts to ultraviolet and strong sunlight; a clear window in the wrong spot triggers partial cure. Opaque drums or UV-blocking film on windows take care of most of the trouble. For those working late, regular indoor lights don’t cause problems, but big skylights need an extra layer of care.
TPGDA ignites at fairly high temperatures, yet it ranks as a combustible liquid. Open flames or sparks in the area spell trouble. Fire marshals favor keeping chemicals like this far from forklifts, tools that spark, or cigarettes. Storing TPGDA with compatible materials is important. Still, I remember once seeing an old chemical store where resins, acids, and oxidizers shared space. One leaky valve could have meant disaster. A proper chemical inventory saves lives and money—no exaggeration.
Drums and totes used for TPGDA have secure lids and inner linings that resist swelling or corrosion. It pays to check seals and gaskets for softness or goop after every delivery. A leak wastes raw materials and makes for a tricky cleanup. Pallets keep drums off concrete, so corrosion from floor moisture gets less chance to creep in.
Chemicals can get along, but low-level mixing with strong acids, bases, or oxidizers invites unwanted reactions. Isolating storage keeps one mistake from turning into a major spill. A spill kit nearby, stocked with absorbent pads and gloves, beats improvising if something goes wrong.
Nobody likes sifting through paperwork, but having labels and Material Safety Data Sheets handy makes real sense. OSHA has strict rules, but I’ve seen labels faded from sunlight or peeled off by rough handling. Good labels mean nobody grabs the wrong drum in a rush, and clear records reassure buyers, regulators, and anyone working in the building.
In short, storing TPGDA sounds simple on paper but calls for vigilance and respect for the material. Controlled temperatures, airflow, careful organization, and strong labels aren’t just rules—they’re lessons learned from people who’ve seen what goes wrong if you cut corners.
Tripropylene glycol diacrylate shows up in a lot of industries. Makers of inks, adhesives, and coatings rely on it to improve how their products cure and stick. Folks working in those plants might get familiar with its sharp, slightly sweet odor. The thing is, even if the name sounds like something out of a chemistry textbook, what really matters here is the straightforward risk it brings: skin irritation, eye damage, and breathing trouble with careless handling. Just because you can’t see fumes doesn’t mean they're harmless. In my early days on a shop floor, I saw a coworker get a nasty rash from rushing to work without gloves. He never made that mistake again. That personal reminder sticks with me and shapes how I view routine safety precautions.
The trouble starts with the liquid itself. Spills soak into gloves or sleeves. Splashing happens during pouring or mixing, and the fumes can hang in the air in tight spaces. Messy workstations raise risks for everybody because one error—like forgetting to close the drum—invites exposure. From experience, it’s not only about what labels say. Sometimes bosses get complacent, stop restocking goggles and gloves, or skip training for new hires. That’s where problems grow roots.
Staying safe around tripropylene glycol diacrylate comes down to wearing real protection, not just relying on company checklists. I learned from old-timers to pick thick, chemical-resistant gloves—not just any latex pair. Safety goggles with side shields help out, especially for anyone opening fresh containers. Proper ventilation reduces the stuff you breathe, so fume hoods and exhaust fans aren’t extras—they’re must-haves.
Over time, those habits turn into muscle memory. I remember keeping a spare change of clothes and extra gloves in my locker, just in case. Eye wash stations and showers aren’t just wall ornaments, either. Quick response stops small splashes from turning into full-blown health scares. Folks looking after each other—reminding a coworker to suit up or fixing a broken vent fan—make everybody safer.
Regulations like OSHA and the European REACH set specific limits and demand regular safety data sheets. The rules exist for a reason, but paperwork won’t stop an accident in the moment. What keeps people safe is everyday respect for the risks. Signing off on chemical deliveries, storing containers out of sunlight, and double-checking the labels—these habits protect more than profits; they shield hands, eyes, and lungs.
Training needs to feel real, not just a once-a-year formality. Bringing in someone who’s seen injuries firsthand hits harder than a slideshow of regulations. Regular drills, gear checks, and open conversations encourage a shop culture where even the newest worker feels unafraid to question shortcuts. I once caught a supervisor using thin gloves to move a leaking drum. Calling it out mattered, not to stir trouble, but to put health before speed.
Good safety doesn’t happen by accident—it comes from respecting the material each time, no matter how routine the job. Keeping clear labels and instructions in the local language, checking expiration dates on protective gear, and installing spill containment kits close to where folks work beat just putting posters on the wall. Investing in the right tools and honest training means fewer surprises and safer shifts.
Tripropylene glycol diacrylate isn’t about to disappear from shop floors or factory lines. As long as companies use it, the smartest workers will set an example: suit up before clocking in, fix problems instead of hiding them, and trust that protecting yourself protects everyone around you.
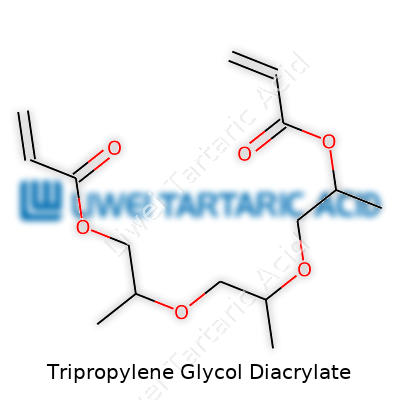
Tripropylene Glycol Diacrylate, often shortened as TPGDA, carries a structure that packs a punch in the world of industrial chemistry. It comes from the family of acrylate esters, which means it forms by attaching acrylic acid molecules to another central molecule—in this case, tripropylene glycol. Imagine three propylene glycol chains joined together, each dangling the acrylate group at both ends. This gives the whole molecule a linear and somewhat flexible backbone, peppered with acrylate groups ready to react.
The chemical formula is C21H34O6. If someone draws it out, they see two acrylate (CH2=CHCOO–) bits stuck on either side of a core built from three propylene glycol units. This setup is no academic trivia—it shapes how the molecule behaves in real-world applications.
TPGDA’s shape isn’t just a curiosity for chemists—its chemical bonds tell a bigger story. Each acrylate group acts like a special reactive hook that links up with others under ultraviolet light, a process called curing. In my own work on resin formulations, switching from monofunctional acrylates to ones like TPGDA makes a true difference. Cure speed jumps and the end result hardens up, all because both acrylate groups anchor in place during the reaction.
This makes hard coatings sturdier and less prone to scratches or yellowing over time. Print shops, automotive plants, and electronics manufacturers look for those perks, which tie back to the structure—a backbone strong enough to lend flexibility, wings ready to lock in crosslinks, and ends eager for chemistry. Having tried sample formulations over the years, adding TPGDA often cuts down tackiness, reinforces tensile strength, and allows smooth blending with other monomers.
Every molecule tells a safety story too. Acrylates, unless cured properly, can irritate skin or cause allergic reactions. TPGDA’s dual acrylate groups give more surface to react, which can make it a bit more likely to provoke sensitivity compared to simpler monoacrylates. I always stress the need for gloves and ventilation based on painful experience—one rushed cleanup job taught me to pay attention. The extra reactivity means all leftovers should be cured out or cleaned up as soon as possible.
Chemicals like TPGDA call for respect along the production line. Labeling must be clear, safety data sheets should live within easy reach, and storage needs a cool, dry environment, away from heat or light sources that could trigger unwanted reactions. Factories owe it to workers to offer solid training in handling acrylates, emphasizing hand protection and fast reporting of spills or skin contact.
Moving forward, I see bigger potential in researching safer analogs or ways to lower exposure. Even improved ventilation systems or on-site curing stations cut down the risk, making workplaces healthier. Substituting raw TPGDA with partially pre-reacted mixtures may also help push the safety needle further. Decades in labs remind me: beneath every formula sits a mix of tradition and evolution, blending great chemistry with everyday safety.