The story of (S)-(+)-1,2-Propanediol stretches back to a time when chemists chased the mysteries of chiral molecules and the ways their “handedness” could change how chemicals work in the real world. Early in the twentieth century, researchers puzzled over how certain compounds, identical except for the way their atoms twist, could have wildly different properties and uses. This specific enantiomer caught the eye of folks working on pharmaceuticals and food, who knew its chirality could make all the difference in safety and bioactivity. Over the decades, manufacturers scaled up methods for making it in purer forms as the demand for chiral-specific compounds grew. The focus sharpened in the late 1970s when separation technology caught up to the ambitions of applied chemistry, letting the biotech, drug, and flavors industries tap into new possibilities.
(S)-(+)-1,2-Propanediol shows up as a clear, almost syrupy liquid with a faint sweet taste and a mild odor. Its roots stretch deep into the chemical industry, but most people brush up against it through its refined roles in food, pharma, and personal care. What sets it apart from its racemic or (R)-(-) counterparts is the direction of the optical rotation and the way enzymes and biological systems respond to it. That difference matters, especially when companies aim for both safety and effectiveness. As chiral purity matters more, this single-handed version now earns a spot on ingredient lists and safety sheets in a way that wasn’t true forty years ago.
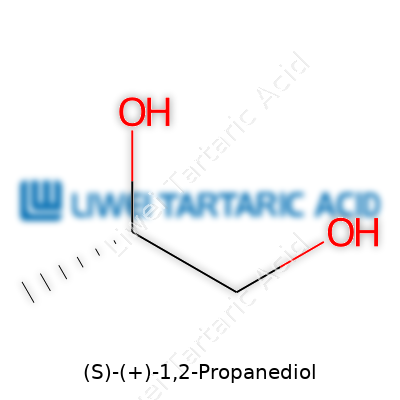
(S)-(+)-1,2-Propanediol carries the formula C3H8O2, and its molecular weight lands at 76.09 g/mol. It dissolves well in water, mixes with most alcohols and organic solvents, and sits comfortably in the ranks of low-toxicity glycols. Its boiling point hovers around 188°C, and it freezes below -60°C. With its specific rotation in the +20° range, labs easily distinguish it from its mirror image. From my time on process floors and in pilot labs, workers appreciate how it resists melting down under normal handling and doesn’t light up under rough storage conditions.
Chemists stick to clear-cut rules to guarantee each batch delivers the right handedness and purity for demanding jobs. Labels highlight enantiomeric excess, typically no less than 99%, since even small drifts can tangle up biological interactions or regulatory reviews. Impurity profiles, residual solvents, and water content land near the top of priority lists for pharma uses. Safety datasheets flag the compound as non-irritating in typical concentrations, and manufacturers include information calling out the exact method used for stereochemical verification. From experience keeping up with audit trails and international guidelines, having these technical specifics right every time is essential for maintaining credibility in tightly regulated sectors.
Factories rely on two main approaches: asymmetric synthesis catalyzed by specialized metal complexes or, more in tune with sustainability, biocatalysis using engineered enzymes or microbial fermentation. Early commercial runs leaned heavily on costly chemical catalysts, which needed expensive purification steps to weed out unwanted forms. Later, bug-based fermentation stepped in, drawing on genetically modified strains of bacteria or yeast. These living factories spin out impressive yields with fewer byproducts, and regulatory bodies often look more kindly on these “green chemistry” routes. With both methods, fine-tuning temperature, pH, and feedstock purity can spell the difference between a batch-winning yield and a high-waste headache.
(S)-(+)-1,2-Propanediol isn’t just content with being itself. It steps up as a building block for more complex compounds: pharma intermediates, plasticizers, synthetic flavors, and chiral catalysts. Chemists modify its alcohol groups to create esters, ethers, or carbonates, each with a custom use on factory floors or lab benches. In one memorable project, I saw a team turn this glycol into a key side chain for an anti-inflammatory drug, taking advantage of its ready reactivity and reliable stereochemistry. The compound also gets oxidized for use in specialty resins, cross-linkers, and optical brighteners, each tweak carefully planned to preserve its handedness.
Across catalogs and safety data sheets, this material answers to many names: (S)-Propane-1,2-diol, (S)-(+)-1,2-Dihydroxypropane, L-Propylene Glycol, (S)-(+)-Propylene Glycol, and sometimes just “chiral propylene glycol” when shorthand rules the day. Each variant name has a champion in a particular industry: pharma engineers tend to stick to the full IUPAC names during documentation, while folks in food or cosmetics might reach for the “L-” or “(+)-” terms when specifying grades.
One of the compound’s strongest points is its low toxicity compared to many industrial glycols, but nobody gets to skip the basics. Workers wear gloves and eye protection, especially when handling raw or concentrated forms, and ventilation keeps vapors in check. In case of accidental spills, (S)-(+)-1,2-Propanediol washes up easily but doesn’t demand hazmat-level fuss. Food and pharma producers comply with international rules: FDA approvals, European E-number labeling, and documentation under ISO or GMP standards. Repeated audits and spot checks back up claims that each drum or bottle delivers what the label promises, an effort that builds long-term trust with regulators and customers.
Everywhere a chemist or formulator needs a chiral backbone, (S)-(+)-1,2-Propanediol stakes a claim. Food flavor houses use it as a carrier and stabilizer for delicate fragrances that can’t stand up to racemic blends. In the pharmaceuticals’ world, it slips seamlessly into formulations for drugs that demand both safety and reliable performance, especially those where stereochemistry can swing potency or liability. Personal care brands work it into moisturizers and serums as a humectant—drawing water without irritating skin or changing the scent profile. Thermoset resin makers and specialty polymer engineers keep it handy as a chiral monomer or crosslinker, capitalizing on its ability to introduce new properties without scrambling established supply chains.
Ongoing R&D treats (S)-(+)-1,2-Propanediol as more than just a commodity—it’s a stepping stone for the next wave of drugs, flavors, and green materials. Universities and corporate labs dive into optimizing fermentation strains, shaving off both cost and carbon footprint. Modifying its structure opens doors to new chiral catalysts, especially now that asymmetric synthesis sits front-and-center in making next-generation medicines. In close discussions with researchers, it becomes clear that subtle changes—making it a little more reactive, or a little less prone to oxidize—can change entire process flows, saving energy or reducing waste.
Early animal and in vitro studies gave (S)-(+)-1,2-Propanediol a relatively clean bill of health, especially compared to related glycols or older additives. Regulatory reviews show much higher safety thresholds for this enantiomer, both in single-dose studies and repeated exposure models. That said, as with any food or pharma ingredient, repeated validation and long-term monitoring edge out old complacency. Labs continue probing for subtle metabolic or allergenic effects, sometimes looking at populations with rare enzyme deficiencies for edge-case risks. Real-world experience over decades points to low toxicity, but vigilance never slips entirely off the agenda in risk management meetings.
Demand for chiral purity in all things—from flavors and drugs to advanced polymers—shows no sign of slowing down, and (S)-(+)-1,2-Propanediol sits right in the crosshairs of this trend. Growing pressure for greener production methods sparks fresh investment in enzyme design and closed-loop fermenters. Regulatory changes, in both labeling and maximum residue limits, light a fire under producers who know customers want to understand every ingredient they put in their bodies. My own experience with both big multinationals and local specialty shops shows a willingness to shift toward safer, more sustainable sources, as long as performance, safety, and cost line up. Looking ahead, chiral glycols like this one will likely anchor innovations in both large-scale manufacturing and boutique, high-value niche products, guided by a belief that the right molecule in the right hand makes all the difference.
People who work in labs making new medicines depend on (S)-(+)-1,2-Propanediol to build complex drugs. Its chiral nature, that is, its ability to distinguish between "left-handed" and "right-handed" forms of molecules, helps researchers develop drugs with fewer side effects. In my years reading life science journals, I’ve seen it make a difference in the synthesis of beta-blockers and certain antivirals. This substance allows chemists to craft molecules that closely match the body’s natural forms, so medications work more effectively and are better tolerated. Safety matters—the FDA and EMA often favor drugs with single-enantiomer forms like those produced using this chemical.
Food companies often use (S)-(+)-1,2-Propanediol as a building block to create safe and stable sweeteners and flavors. For people who care about what goes into their drinks or chewing gum, this compound helps keep gums moist and flavors balanced. I’ve spoken with flavor chemists who rely on its predictable performance during flavor formulation, especially for products that must taste the same every time. Unlike synthetic additives, this chiral glycol supports “natural flavor” claims, since it can be sourced from renewable resources and fits safety standards set by food regulators.
Many cosmetic companies have turned to (S)-(+)-1,2-Propanediol to avoid controversial alternatives like propylene glycol from petroleum. Skin creams, serums, and lotions stay smooth and easy to apply because this ingredient keeps water locked in and prevents crystallization. Having lived with sensitive skin, I know how important it is to avoid irritants. (S)-(+)-1,2-Propanediol offers a genuine option that feels gentle, and dermatologists tend to trust it due to its lower risk of triggering allergic reactions compared to other glycols.
Specialty chemical companies use (S)-(+)-1,2-Propanediol as a solvent in electronics, cleaning agents, and printing inks. Its advantage over more volatile or toxic solvents like methanol shows up in reduced worker exposure risks and fewer disposal headaches. Industrial safety teams favor it; I heard from a compliance officer who called it a “workhorse” for shifting operations toward greener manufacturing. Fewer fumes and better biosafety records can help protect workers and communities.
Continued research from universities and the chemical industry aims to produce (S)-(+)-1,2-Propanediol from non-fossil sources like corn or sugar beets. These bio-based methods can lower overall greenhouse gas footprints for finished products. Some researchers, including several teams in the US and Europe, are working on engineered microbes to generate this compound more efficiently. As demand rises from pharmaceutical, food, and cosmetic brands looking to clean up their supply chains, the pressure mounts to invest in renewable raw materials and streamline production.
People today want to know the origins and safety of what they consume or apply on their skin. Brands that disclose the use of (S)-(+)-1,2-Propanediol and back up their claims with reliable sourcing and third-party certifications build stronger reputations. Many health-conscious shoppers—myself included—are swayed more by a well-explained label than trendy marketing. Responsible sourcing and open labeling encourage safer choices across industries.
If you ever looked at a label and noticed a chemical name you can’t pronounce, you’re not alone. (S)-(+)-1,2-Propanediol might sound intimidating, but its close cousin, propylene glycol, pops up in ice cream, medicine, and cosmetic products all over the world. The (S) isomer draws attention in scientific circles because of its natural chirality and its presence in some metabolic pathways. Many folks wonder if it really deserves a place in food or pharmaceutical products.
My background in biochemistry means family and friends sometimes hand me ingredient lists to interpret. Several governing bodies have examined (S)-(+)-1,2-Propanediol, including the FDA and European Food Safety Authority. Both referenced studies in animals and humans showing this molecule breaks down into lactic acid and pyruvate, which are already found in our bodies. People tend to handle reasonable dietary amounts just fine.
Researchers tested exposure limits extensively in rodents, with typical safety numbers far above anything a person would eat or swallow in medication. The FDA categorizes the racemic compound — which includes both (S) and (R) forms — as “Generally Recognized as Safe” when used in limited amounts.
One standout fact: this molecule is far less toxic than something like ethylene glycol, which shows up in antifreeze. News stories sometimes mix the two, causing unnecessary alarm. Studies on (S)-(+)-1,2-Propanediol do not show the same risks, especially in the levels used in consumer products.
Eating or drinking too much of anything can cause problems. With (S)-(+)-1,2-Propanediol, extremely high amounts may stress the kidneys or liver in sensitive people, usually those with pre-existing issues or infants. Rare allergic reactions sometimes make the news, but these cases look like outliers. In my own experience helping patients, complaints about this ingredient almost always come from confusion with propylene glycol allergies or vague advice from the internet.
Pharmaceuticals sometimes use (S)-(+)-1,2-Propanediol as a solvent to help drugs dissolve better. This can improve effectiveness and dosage control, especially for children’s medicine. Since kids can process chemicals differently than grown-ups, pharmaceutical companies keep intake levels below thresholds established by international guidelines. Doctors monitor vulnerable patients to prevent any side effects.
People deserve to know what’s in their food and medicine. One thing industry players should work on: plain language explanations and honest communication. If companies share how much (S)-(+)-1,2-Propanediol goes into products, how safety research gets done, and why these choices are made, it builds trust.
Regulators have the job of checking company data, and consumer watchdog groups often conduct their investigations. Both help catch mistakes and make sure manufacturers don’t overstep limits. In my lab days, we often fielded calls from food safety inspectors — the back-and-forth helped catch small problems before they grew larger.
No one chemical will ever please everyone. Still, decades of research and careful oversight suggest that (S)-(+)-1,2-Propanediol can be safely included in the amounts typically used in food and pharmaceuticals. More ongoing research solidifies this confidence and encourages manufacturers to keep refining their formulas so products remain both safe and effective for a broad range of people.
Everyday chemistry rarely gets headlines, but the handedness of molecules—something most people miss—matters a lot, especially with substances like 1,2-propanediol. A molecule shaped like your left hand acts differently from the right, even when they share the same recipe of atoms. (S)-(+)-1,2-Propanediol, a chiral molecule, provides a solid example. It has a twin, (R)-enantiomer, plus a racemic mixture that blends both forms. This simple detail has serious consequences in real life, especially where health, food, and science cross paths.
Ask anyone in the pharmaceutical industry, and you’ll hear that chirality shapes a drug’s fate. The body reads each enantiomer as a slightly different puzzle piece. Mix the (S)- and (R)-forms together, and you don’t always get the same results as you do with a pure form. I remember learning in lab courses how adding a racemic blend into a biological system turned up different enzymes responses and unexpected biological activity than with a single enantiomer. The (S)-(+)-form can fit into one enzyme, while the (R)-type bounces off. That’s a small shift in chemistry with outsized power.
Industry uses 1,2-propanediol as a solvent and humectant. In foods and flavors, enantiomers give off different tastes and smells. I once handled a batch for a flavor company; the aroma of one form registered as sharp and sweet, but the other carried an oily, flatter note. That’s no accident—taste buds and olfactory cells act like locks built for a specific orientation. Regulatory authorities, like the US FDA, require safety profiling to distinguish the forms, especially since (S)-(+)-1,2-propanediol is considered safer for food and pharmaceutical use compared to its sibling. When racemates slip into the mix, carefully tracked labelling protects consumers and manufacturers from missteps.
Isolation of a pure (S)-enantiomer once posed a technical roadblock. These days, advances like enzymatic and chiral column separation allow greater control. Production companies rely on these methods because the wrong enantiomer sometimes brings unintended effects. The pharmaceutical world learned tough lessons from medicines like thalidomide, where one enantiomer soothed pain, while its mirror version caused birth defects. Even outside medicine, regulations kick in—European guidelines often require documentation on which form has been used in products from vape liquids to food flavorings.
It’s tempting to treat all molecules as the same, but real progress—and safety—depends on paying attention to details like chirality. By embracing newer purification techniques and stricter quality controls, companies lower the risks that come with mixing up these siblings. From the lab notebooks to food shelves, the difference between (S)-(+)-1,2-propanediol and its racemic or (R)-enantiomer shines brightest in the careful hands of those who refuse to accept “close enough.” That’s where science works best, honoring the twists and turns of molecules as much as any genetic code.
I’ve handled plenty of chemicals in my own lab time, and (S)-(+)-1,2-Propanediol has always stood out for its versatility. Chemists reach for it in synthesis, pharma, even as a chiral building block. It feels easy enough to treat it like just another clear, syrupy liquid, but trouble often hides in familiarity. Sticking to some straight-up safety habits, every time, makes the difference between a good day at work and a trip to the hospital.
(S)-(+)-1,2-Propanediol might not light up or kick off vapor like some of its relatives, but skipping the basics can lead to headaches nobody wants. Store it somewhere cool—ideally below room temperature—since heat will boost degradation. In my experience, placing chemicals on high shelves near windows feels convenient until summer turns your storeroom into an oven. Sunlight speeds up breakdown and pushes impurities into your product; keep it in the dark, in a sturdy, tightly closed bottle, away from incompatible stuff like strong oxidizers or acids.
Humidity rarely gets much attention, but I’ve seen water slowly sneak into containers with a loose cap or an old seal. Even a small drip dulls purity over time. I suggest using a dry, sealed environment. Plain glass works well, but for large volumes or long-term storage, you’ll want specialty bottles, likely made of high-quality plastic, that keep the contents isolated. Check that label every time—shelf-life and batch numbers give away if things are going south before you notice by eye.
Handing out the right gear sits at the core of lab safety. Gloves and goggles shouldn’t be optional. Splash-resistant lab coats keep skin out of harm’s way, and a chemical fume hood saves lungs from the rare but possible vapor that escapes. Spills tend to happen when people rush. I always double-check container lids and never pour straight from a bulky drum. Use a clean spatula, never mix with food or drink areas, and never assume a colorless liquid poses zero threat. Inhalation or skin contact can irritate, though symptoms may show up late.
Disposal comes up less often but deserves just as much focus. Don’t wash it down the drain or toss it into trash bins. Used products need collection in labeled containers until a certified waste company gets involved. Local regulations vary, so check before moving forward.
Fumbling with chemicals rarely ends well; habits build culture, and culture builds trust. Over the years, I’ve seen teams cut corners when work piles up. It makes sense—not everyone feels danger lurking behind a common name like propanediol. But that’s exactly why talking about these habits, setting rules, and retraining on best handling practices keeps the risks low. Experienced coworkers teach methods that stick, like keeping written logs for opening dates and maintaining checklists for every chemical you use.
Safety comes from doing the simple things right, every time. Good storage and sound handling aren’t just compliance—they’re signs of respect for your team and the work. Ordinary vigilance pays off with consistent results and a workday free of emergency showers and eye-wash stations.
Stepping into any chemical supplier’s catalog, it’s easy to get lost in percentages, grades, and options. (S)-(+)-1,2-Propanediol, a specialty chemical with a valuable chiral center, tells a familiar story. Purity snapshots in brochures might promise the moon, but what do buyers really get? From bench scientists elbow-deep in asymmetric synthesis to QC analysts watching for rogue peaks, purity isn’t just a checkbox — it drives everything from reaction yields to patient safety in pharma.
Most suppliers roll out (S)-(+)-1,2-Propanediol at a stated purity level between 98% and 99%. The big question: is this just a glossy number, or does it hold up in real-world use? Price points reflect these purity brackets. It’s the default for most chemical suppliers, a sweet spot balancing production cost, purification headaches, and wide application. Some companies will stretch for stereochemical “enantiopurity,” reporting enantiomeric excess (ee) values above 98%, sometimes hitting 99% or higher. This catches the eye of fine chemical producers and pharmaceutical teams, who can’t afford side-products making it into clinical batches.
Occasionally, you’ll see ultra-high-purity product, 99.5% or more, always at a hefty premium. These rarer products often head toward analytical labs or high-demand pharma plants. I’ve purchased such material for chiral auxiliary studies — the extra cost can be worth every cent if your results ride on trace-level impurities. Tighter specs give insurance that unexpected contaminants won’t derail a synthesis or distort bioassay data. The label may say “optical purity ≥99%,” but real performance depends on storage, shipping conditions, and batch-to-batch consistency.
An extra 0.5% impurity level doesn’t seem like much, but it writes the difference between a straightforward scale-up and months troubleshooting side products. Contaminants might derail catalysts, spike toxicity in a biological screen, or provoke regulatory headaches. If you’re developing a new route or optimizing for yield, even low-level racemates or solvent leftovers force extra steps for cleanup — chewing through budget and calendar days.
Pharmaceutical workflows put the spotlight on both chemical and enantiomeric purity, since both impact safety and regulatory approval. One batch shipped with 1% racemization can cost months of validation work or, in a worst-case, patient safety. On the other end, industrial users putting this compound into resins or additives might accept a lower grade, shining a light on those 98% products. It’s all about how much risk you’re willing to tolerate, weighed against price and downstream impact.
Better purification starts with careful process design: fractional distillation, chiral chromatography, and rigorous in-process testing keep impurity levels in check. Suppliers who invest in traceability, transparent quality control data, and batch-specific certificates make life easier for buyers. Every researcher benefits from asking for detailed chromatograms, not just relying on a technical data sheet percentage.
Some labs double-check with their own GC or HPLC systems, picking up what a supplier’s quick-check might miss. There’s also growing hunger for tight QA partnerships with suppliers, building relationships that allow for custom purification runs and more consistent batch quality.
Relying on sticker purity figures leaves too much to chance. Deciding what level is truly “pure enough” means knowing the stakes in your synthesis, talking candidly with your supplier, and sometimes laying out for higher grades. It’s a lesson that pays dividends in fewer headaches and better science, every time.