Chemistry traces a winding path through history, and (R)-(-)-1,2-Propanediol reveals a story shaped by curiosity and the drive to build new tools. Early researchers in the late 1800s looked for ways to separate and understand chiral molecules, driving discoveries in stereochemistry. In the decades that followed, advancements in synthesis methods allowed labs to produce enantiomerically pure forms of this molecule, stepping away from crude racemic mixtures. This progress opened doors for a host of industries that came calling for chiral agents. Over time, demand for enantiomerically pure (R)-(-)-1,2-Propanediol grew, especially as pharmaceutical makers spotted the need for chiral building blocks that meet tighter regulatory demands. Steady investment in green chemistry and biocatalytic processes during the late twentieth and early twenty-first centuries strengthened both environmental awareness and the accessibility of pure material. Key publications and patents from academic groups and major manufacturers have left a wide paper trail, marking real progress toward cleaner processes and higher yields.
(R)-(-)-1,2-Propanediol comes off as an unassuming molecule—clear, slightly viscous, with not much in the way of odor. But beneath this simplicity lies a structure that’s become indispensable across chemical manufacturing, pharmaceuticals, and specialty chemical production. With one chiral center, the (R)-enantiomer supports synthesis work that delivers single-isomer drugs and specialty intermediates where chiral purity determines product safety and effectiveness. This propanediol acts as a stable, manageable input for both batch and continuous production scales, suiting both research quantities and bulk orders in industrial settings. Formulators in cosmetic and flavor industries have turned to this chemical as a low-toxicity humectant and carrier, favoring it over racemic mixes for sensitive applications.
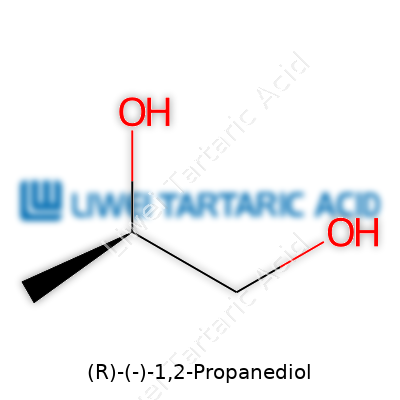
(R)-(-)-1,2-Propanediol presents itself as a colorless, hygroscopic liquid at room temperature. Molecular formula C₃H₈O₂, molecular weight about 76.09 g/mol, its optical activity sets it apart—[α]D of -32° (neat, pure sample). The chemical boils at 188°C under atmospheric pressure and melts around -59°C. Water and alcohols mix freely with it, which means blending and dissolution cause few headaches. Density hovers near 1.036 g/cm³. The secondary alcohol group at carbon two and the primary at carbon one invite further reactions, suiting both reduction and oxidation steps in organic protocols. The hydrogen bonding properties create a distinctive solvation environment, let’s say, for enzymes and active ingredients in both pharma and cosmetic formulations. Viscosity and volatility remain low enough to handle with common pumping and transfer setups without high evaporation loss, even in large tanks.
Industrial supply chains now rely on tight standards for (R)-(-)-1,2-Propanediol. Most reputable vendors ship products boasting greater than 98% enantiomeric excess and typical purity levels not lower than 99%. Certificate of Analysis will usually report water content, residual solvent traces, and any possible byproducts from synthesis such as propylene oxide. Labeling covers the chemical name, CAS number (4254-15-3), UN number for transport, and a run of batch information matched by documentation that works for global regulatory checks. Good manufacturing practice and ISO certificates drive trust—traceability back to the production line means quality managers don’t lose sleep over off-spec shipments. Packaging comes in sealed containers ranging from amber glass bottles for smaller quantities to galvanized steel drums or HDPE barrels for bulk, each labeled with clear hazard diamonds as called for under GHS.
Older generations of chemists made (R)-(-)-1,2-Propanediol using resolution from racemic mixtures, yet this proved low-yield, wasteful, and time-consuming. The real leap forward arrived with advances in enzymatic and microbial fermentation. Microbial reduction of hydroxyacetone, typically using engineered strains of yeast or bacteria, became a key route. Using biocatalysts, plants now produce enantiomerically pure product far more efficiently, side-stepping the pile-up of unusable isomer and bypassing heavy metal catalysts that created headaches for waste disposal. Several chemical reduction pathways persist for specialty markets, such as asymmetric hydrogenation of acetol with chiral ligands, but these see less action due to greater cost or complexity. In my own experience, moving a research project from racemic to biocatalytic routes dropped solvent waste by more than half, while downstream purification—chromatographic or crystallization—tightened product quality and reduced headaches during scale-up. Process chemists now run pilot batches openly discussing life-cycle impact, combining performance with a lower environmental load.
Chemists know (R)-(-)-1,2-Propanediol as a two-faced companion in the lab—both as a solvent of unique properties and as a building block for downstream chemistry. The vicinal diol group opens doors for acetal formation, oxidation to hydroxyacetone or lactic acid derivatives, and conversion into epoxides or carbonates under the right catalytic setups. Protecting group strategies draw on this backbone to tether, suspend, and later reveal functional handles on high-value molecules. When working on scale, the combination of primary and secondary alcohols lets manufacturers steer outcomes based on simple reaction conditions: acidic settings trigger dehydration, mild oxidants favor selective transformations, and reductive workups open other windows. Direct modification extends into polymer chemistry, with functionalization for biodegradable polyesters fast becoming part of green plastic research. Biocatalytic modification opens further possibilities, where site-specific oxidations can produce short-lived intermediates tough to prepare any other way.
Suppliers and researchers toss around different names for (R)-(-)-1,2-Propanediol depending on region and end use. “(R)-Propylene glycol” or “(R)-1,2-Dihydroxypropane” turn up in some catalogs. Older pharmacopoeias cite “(R)-Propane-1,2-diol” or “L(-)-Propylene glycol”. For routine shipments, trade names reflect source more than chemical detail, and technical data sheets pin down specific catalog numbers to single enantiomers. Matching names across markets sometimes involves old EC or EINECS numbers, especially for documentation in Europe and Asia. In pharmaceutical and food chain supply lines, stringent compliance rules highlight the need to spell out enantiomeric purity on every formal document.
Although the safety profile of (R)-(-)-1,2-Propanediol rates better than many chemicals once considered for similar roles, production and use still demand real caution. Inhalation risk stays low at room temperature, but atomized droplets from mixing or spraying need tight ventilation and protective equipment. Spills call for prompt containment since the compound’s high water solubility lets it spread fast across industrial floors. Well-run laboratories emphasize eyewear, nitrile gloves, and good engineering controls—especially during large-scale transfer. Storage best practices recommend tight seals and stable, temperate conditions to avoid unwanted hydrolysis or microbial growth. Regulatory guidance from OSHA and EU REACH require regular training and hazard communication for anyone developing or using this chemical above research scale. Waste bottles merit care; although toxicity risks lag behind glycols like ethylene glycol, improper disposal can still contaminate groundwater or upset wastewater treatment.
Few molecules earn as broad a reach as (R)-(-)-1,2-Propanediol. In pharmaceuticals, this material serves as a chiral intermediate in the synthesis of optically pure APIs, especially beta-blockers and anti-infectives, where control of enantiomeric outcome steers both safety and performance. Specialty polymer makers use it as a monomer in biodegradable plastics, answering demand for alternatives to petroleum-based resins. Food and cosmetic companies tap its low sensitization potential and pleasant skin feel as a carrier, humectant, and stabilizer. In lab research, I’ve used it as both a solvent and cryoprotectant, for enzyme storage and plant tissue work—flexibility not easily matched by more “standard” alcohols. Technical fields including analytical chemistry and separation science add it to chiral mobile phases for improved selectivity. Rapid growth in the flavors and fragrances sector points to a stable market, with more work focused on flavor carriers and safe cosmetic bases.
Lab groups and industrial teams still invest serious time into improving production of (R)-(-)-1,2-Propanediol. Process chemists keep experimenting with new biocatalysts and engineered microorganisms that boast better selectivity and higher substrate loading, trying to cut production costs and shrink environmental impact. Research has also traveled toward continuous flow methods, using microreactors to sharpen yields and reduce byproduct formation even at plant scale. Work in chemical modification targets new derivatives and conjugates—especially for high-value pharma intermediates and as building blocks in advanced polymer design. Analytical chemists sharpen control of enantiopurity through powerful chiral chromatography and online monitoring, so that every shipment reaches its destination meeting ever tighter specs. There’s also a stream of patent activity around direct fermentative production of advanced derivatives, hoping to bypass steps that traditionally called for toxic reagents or harsh reaction conditions.
Toxicologists keep a close watch on all glycols for their breakdown in the body. (R)-(-)-1,2-Propanediol, like its racemic cousin, usually shows up as a low-toxicity choice. Oral and dermal exposure studies in animals show few acute effects, and chronic dosing hints at minimal bioaccumulation. For human use, regulators in both pharma and food sectors set strict intake limits based on large metabolic studies. Still, not every unknown has been nailed down, especially as this compound shows up in more finished products. In my own work, after sharp-topped spills or accidental splashes, medical monitoring never turned up measurable harm, but regular safety reviews point to the need for careful handling and record-keeping. Ongoing research looks for subtle toxicity or allergenic responses over longer exposure or in vulnerable populations, making vigilance an everyday priority.
The road ahead for (R)-(-)-1,2-Propanediol winds through several expanding fields. With biodegradable plastics and green solvents in high demand, more companies commit to biosourced and renewable propanediol, using fermentation instead of fossil inputs. Advanced chemical manufacturing prizes optical purity ever more, as single-enantiomer drugs become the standard and regulatory bodies insist on proof of performance and safety. Analytical techniques kept pace, allowing producers to tighten specs and reach new markets—sometimes before consumer demand even comes into view. Research into new chiral catalysts and improved engineered microbes points toward higher yields, faster batch turnover, and softer footprints all along the supply chain. Direct conjugation work stands ready to debut new derivatives that feed innovation in pharmaceuticals, polymer science, and sustainable product design. Each advancement builds on work already logged by thousands of chemists and engineers in labs small and large, laying groundwork for safer, smarter, and more sustainable industrial chemistry.
(R)-(-)-1,2-Propanediol isn’t really a household name, but for chemists, pharmacists, and manufacturers, it pops up in more than a few toolkits. Its chemical quirks lean on chirality—it’s one of those compounds that has a mirror-image sibling, which means the world cares about which “handedness” you choose for a specific application.
The pharmaceutical world favors pure, single-handed molecules. That left-handed or right-handed difference can shape how a drug works inside the body. I’ve seen drug makers rely on (R)-(-)-1,2-Propanediol as a starting point for creating medicines where the three-dimensional structure matters. Chirality isn’t just chemistry trivia—it affects how the body reacts. A subtle change in shape might turn a useful medicine into something inactive or potentially harmful. So, when the goal is precision and safety, the value of a chiral building block like (R)-(-)-1,2-Propanediol becomes obvious. Companies have used it for synthesizing beta-blockers and antiviral agents, where mere milligram differences in molecular “handedness” change the final pill’s safety.
Food scientists and flavor makers turn to (R)-(-)-1,2-Propanediol as well. In these labs, taste matters, and so does purity. Some flavors twist and change depending on the molecular orientation—the wrong enantiomer can make a flavor bitter or even unsafe. Using (R)-(-)-1,2-Propanediol, formulators reproduce natural flavors that fool the tongue in the right way. The compound isn’t widespread in food itself, but its presence behind the scenes lets companies meet strict standards set by regulators.
Organic chemists love a reliable chiral backbone. I think back to graduate school syntheses, where efficiency meant fewer steps, less waste, and higher yields. (R)-(-)-1,2-Propanediol works as a chiral auxiliary or an intermediate, making it easier to produce specific molecular shapes that downstream products require. I’ve seen it used in labs that specialize in creating fine chemicals—substances with just the right twist to fit together with others in a multi-step process. It can shave weeks off a project timeline if the starting materials point the whole synthesis in the desired direction.
Cosmetic chemists watch for skin reactions and product stability. (R)-(-)-1,2-Propanediol makes its way into certain skin creams and lotions, especially where manufacturers seek mildness. Some companies tout chiral purity for sensitive skin products, because a pure enantiomer sometimes leads to gentler formulations. For folks with allergies or finely tuned skin, this choice isn’t small. As someone who’s fielded calls from friends with sensitive skin, I see the importance of picking raw materials that are both safe and effective.
Governments keep a close eye on chemicals that touch food, medicine, or skin. (R)-(-)-1,2-Propanediol has to meet tough purity standards, especially since its cousin, racemic 1,2-propanediol, shows up in antifreeze and other industrial products. Purity testing, documentation, and traceability drive up costs, but these safeguards lower risks. I’ve dealt with regulators asking for transparent sourcing and clear records, and it’s clear the push for safer, traceable ingredients isn’t slowing down. Cleaner chemistry remains the best path for the future.
Manufacturing chiral compounds isn’t always gentle on the planet. Energy use, solvents, and waste pile up. Green chemistry efforts now aim to make (R)-(-)-1,2-Propanediol using less energy, renewable materials, and fewer hazardous steps. Enzyme-based manufacturing, for instance, lets companies trim their carbon footprint while keeping enantiomeric purity high. As demand for sustainable processes grows, companies leading on greener routes stand to gain both market trust and more efficient production.
Ask anyone who’s worked in a laboratory or a pharmaceutical plant: the words “purity grade” can make or break results. In the case of (R)-(-)-1,2-Propanediol, you don’t get much wiggle room. This chiral diol plays a key part in synthesizing drugs, making flavors and fragrances, and creating specialty chemicals that must meet tight quality standards. Anything less than that and the risk of skewed experiments, impurities in products, and failed regulatory checks suddenly starts haunting you.
Most researchers look for (R)-(-)-1,2-Propanediol with at least 98% purity. This isn’t just a preference. Imagine a chiral building block where even small percentages of the S-enantiomer—or unknown byproducts—could trigger a cascade of unintended behaviors. That’s the kind of error no one wants in a pharmaceutical or specialty chemical. My own time running test batches of chiral catalysts told me, the moment you cut corners on source material, side reactions pop up, and performance data starts wobbling.
High-performance liquid chromatography and polarimetry sort out the truth fast. Analytical methods provide a hard look at both purity and enantiomeric excess. Most suppliers stamp their chromatograms on certificates of analysis, calling out figures like “≥99% purity” and “ee >99%.” Some labs prefer even tighter specs, especially in research leading up to regulatory submission. If you’ve chased purity in a project deadline crunch, you know only too well how even a half-percent of extra isomer can put months of work in limbo.
Nobody likes reading recalls on active pharmaceutical ingredients or processed food additives. In both these cases, even trace impurities send up red flags with regulatory agencies like the FDA or EMA. The World Health Organization’s drug standards don’t make exceptions here either. Slipping a poorly-documented chiral ingredient into a process can throw safety, shelf life, and legally-mandated quality out the window. Plenty of small companies start off thinking a “research grade” bottle will do, only to run into trouble with agencies delaying approval because of questionable supply chain documentation.
From my own job sourcing this compound, I’ve watched companies spend more on third-party purity verification than on the actual product to avoid these headaches. No one wants their drug rejected because of a trace contaminant.
Sometimes you run up against issues even buying from reputable sources. Even big vendors turn out batches with minor contamination or inconsistent chiral ratios. Sometimes it’s residual solvents; sometimes it’s a shadow of another enantiomer. Routine spot-checking—pulling HPLC samples from every batch—has become standard practice because everyone knows paperwork alone can’t guarantee what’s inside a barrel.
The route of synthesis makes a big difference. Enzymatic processes and selective reduction pathways tend to deliver higher optical purities. Chemical reduction sometimes leads to traces of the unwanted enantiomer. For truly critical applications—clinical trials, regulatory submissions, precision flavor compounds—you lean into those suppliers who support batches with solid documentation of their purification and enantiomeric enrichment steps.
Purity demands vigilance—not just from analysts, but across research, purchasing, and regulatory teams. Investing in robust supply chain traceability, and nudging suppliers toward better and more transparent testing, lifts quality for everyone along the chain. It’s not just academic; every decimal point in reported purity can mean the difference between a breakthrough and a recall.
(R)-(-)-1,2-Propanediol doesn’t threaten like some volatile chemicals, but careless storage can still create real problems. Many folks approach storage with a casual attitude, thinking of it as just another glycol. Yet this substance can degrade under the wrong conditions, lose its integrity, and put users or lab animals at risk if contamination creeps in.
Years ago, during a stint in a small biotech lab, I watched dozens of reagents lose potency because someone put economy above caution. Local news barely blinked, but every research result lost reliability for months. The lesson felt personal—poor storage is not just a technicality, it shakes confidence in results and wastes time.
This chemical keeps stability at room temperature, so the urgency many feel for refrigeration isn’t required unless a project calls for extreme purity or long-term shelf life. Cool, dry areas protect against moisture and heat, which can cause discoloration or unwanted reactions.
Every chemical shelf should have containers with tight caps. Even a short period with the lid loose can allow air or dust inside leading to impurities and, in turn, unpredictable behavior in research or manufacturing. A plastics cabinet with a double seal has always served well in my experience. That keeps most air out and cuts down the odds anything drips in from above.
Direct sunlight does no favors here. Ultraviolet light can break down many organic chemicals, (R)-(-)-1,2-Propanediol included. Tucking containers in a shaded storage room, away from window light, holds the line for purity and shelf life. Don’t place it near a heater, steam lines, or anywhere prone to temperature swings—consistency is key.
Mix-ups don’t just annoy lab techs, they produce costly setbacks. A friend’s university lab once ruined weeks of work because a flask hadn’t been labeled after filling. Always mark containers clearly with chemical name, date received or prepared, and the responsible technician’s initials. This habit eliminates confusion, especially if turnover in the lab or storeroom runs high.
Stacks of open glassware usher in cross-contamination. I’ve found it’s worth investing in screw-cap bottles made of high-density polyethylene. Glass works well, but if the workspace isn’t climate-controlled, plastics resist breakage in case of a knock or drop.
Bulk delivery brings a new layer of risk. If you’re moving drums or larger containers of (R)-(-)-1,2-Propanediol, keep pallets in clean, dry, and well-ventilated storage. Open and inspect shipments promptly for leaks. I once saw a delivery where a pallet load sat on a damp warehouse floor—within a week, labels had degraded and container bases gave out.
Proper documentation should stick with each container, making it easier to trace the lot source if a problem ever crops up. For safety, make sure a spill kit sits nearby and staff stays trained for clean-up protocols.
Science never stands still, so storage guidelines keep shifting as new studies highlight risks or reveal safer solutions. Anyone handling (R)-(-)-1,2-Propanediol will help themselves by checking current Safety Data Sheets and by seeking advice from colleagues who’ve handled the substance for years. Mistakes tend to happen when routines start to slip, especially during busy spells or staffing changes.
A bit of effort in storage saves more than money and materials. It preserves trust in data, safety for everyone nearby, and respect for every hour spent in the lab.
Spend a day at any research lab or manufacturing plant, and you see how the size of a chemical container changes the flow of work. With something like (R)-(-)-1,2-Propanediol, different users want different amounts. Academic researchers often look for smaller bottles, sometimes just a few hundred milliliters, to run small-scale tests. Chemical companies, on the other hand, might buy the same substance in drums or bulk containers to keep production moving without frequent reorder headaches.
My time spent in a quality control setting showed me the headaches that come with using the wrong package size. Too much product sitting on the shelf risks going bad or being wasted, especially when working with something sensitive to contamination. Ordering too little, on the other hand, stalls projects. Teams end up juggling their schedules, waiting on shipments. That’s money lost and trust in supply lines eroded. The flexibility in packaging helps keep routines smooth for teams of all sizes. Nobody wants to crack open a 200-liter drum of a chemical just to measure out a spoonful.
Smaller packaging doesn't just make life easier for those working at the bench; it also helps maintain safety. Large drums stay in storage areas with proper ventilation, while small bottles or canisters bring the chemical to where it’s actually needed. That helps reduce spill risk and limits the mess if an accident happens. Labeling standards remain clearer for smaller containers, making it harder to mix up what’s in each bottle. Guidelines from agencies like OSHA underline the value of proper labeling and container management in reducing chemical mishaps.
Consistency carries real weight for pharmaceutical and biotech companies who buy (R)-(-)-1,2-Propanediol. Once a big container is opened, exposure to air and moisture can change what’s inside. Packaging smaller amounts lets buyers use what they need and keep the rest sealed, which helps preserve the substance’s properties over time. This plays a role in meeting the strict standards set by organizations like the FDA. Reliable supply in different packages can also help make traceability easier—a valuable safeguard when every batch of product needs to tie back to its source.
Suppliers who offer a range of sizes keep themselves in the conversation with a broader group of customers. They can support everyone from a student learning the ropes in a college lab, to a major producer developing life-saving medication. Paying attention to the demand for varied quantities isn’t just a marketing trick; it’s about supporting scientific progress and safe production across the board. Chemical distributors are listening and responding, which strengthens the value chain for everyone involved.
Customers benefit from talking with suppliers about their actual needs rather than settling for whatever’s left in the catalog. Transparency about shelf life, purity standards, and storage requirements for the package size chosen helps everyone stay on the same page. In my own experience, switching to the right container size meant fewer wasted resources, faster processes, and safer workplaces. Focusing on practical details like package sizing is more than a logistics issue—it’s a foundation for trust, efficiency, and progress in research and industry.
Working with chemicals always asks for presence of mind and respect for the material. (R)-(-)-1,2-Propanediol doesn’t sound as scary as some other names, but it still asks for care on the job or in the lab. Some properties are easy to overlook until an accident draws attention. This colorless liquid shifts from friend to hazard if splashes get on your hands or accidently meet your eyes.
In my own work, training day hammered in glove use—not the floppy ones from a supermarket, but gloves rated for chemical resistance. Good safety glasses make a difference, too. Maybe you’ve felt that sting from a small chemical splash in the past—once is enough to remember a face shield helps. Cotton lab coats also serve to keep skin covered even in warm labs. Busy days tempt folks to cut corners, but those quick fixes pave a rough road.
Nobody wants a shelf accident. I’ve seen clean-up from cracked bottles and sticky benches slow work for everybody in a hurry. Labeled, tightly-sealed containers set on low shelves drop the odds of a spill. Keep this compound away from heat sources and open flames. Most people assume only solvents burn easily, but nearly every chemical needs a fire plan. (R)-(-)-1,2-Propanediol can form flammable fumes if heated enough.
If a spill happens, fast action based on training goes further than panic. Small spills get wiped with absorbent materials—paper towels work as long as you wear gloves and toss everything in an appropriate waste bin. Ventilate the work area by opening windows or starting a fume hood. For larger spills, only a team with chemical spill kits gets the job done without raising risk. I saw a rookie forget to wash up after cleaning small drips; washing with soap and water cut down irritation or rashes.
Prolonged breathing of vapors or direct skin contact with (R)-(-)-1,2-Propanediol may cause mild irritation. Even if nothing feels wrong right away, low-level exposure racks up over weeks. Airflow matters. Open windows and fume hoods help push away anything airborne. I worked once in a lab without a working hood—trust me, you feel the difference later.
Folks new to using this chemical benefit from walking through the safety data sheets before starting work for the day. Signs at the bench, reminders about washing hands, and regular safety drills keep good habits fresh. Setting expectations for what to do if splashed or exposed makes for quick response instead of guessing.
Dumping leftover chemicals or cleaning rags down a regular sink poses risk to more people than just the lab crew. Waste should go in the designated chemical waste containers. Signs should make it obvious where to put contaminated gloves and absorbent wipes, so nobody takes shortcuts on a busy day.
As someone who’s handled different chemicals over the years, it’s clear that small efforts—reading labels again before use, practicing spill response, or simply double-checking storage—cut way down on accidents. Teams that talk safety and build routines together keep everyone a little safer. This doesn’t just help inside the lab; neighbors and communities count on us to keep hazardous materials out of water and air.
| Precaution | Why it Matters |
|---|---|
| Proper gloves and goggles | Protects from skin and eye irritation |
| Chemical-resistant lab coat | Prevents clothing contamination and direct skin contact |
| Good ventilation | Reduces inhalation risk |
| Immediate spill clean-up | Limits spread and reduces exposure |
| Use proper waste containers | Keeps chemicals out of public water and landfill |