Chemical production walks an interesting path. In the 1980s, refinements in propylene oxide processing let chemists unlock new solvents with improved profiles. Propylene Glycol Monomethyl Ether Propionate (PGMEP) arose during a growing need for safer, high-boiling-point solvents, especially in coatings and electronics. Markets needed a replacement for some older, more hazardous glycol ethers. Chemical manufacturers responded through better catalytic routes and distillation techniques. During my early years in labs, shifting away from ethylene-based solvents illustrated a trend toward worker safety and environmental awareness. Demand pushed major producers to ramp up capacities, shining a light on the importance of propylene-based glycols.
PGMEP serves as a colorless, flammable liquid, appreciated for moderate evaporation rates and low odor. Chemists and painters choose it because it bridges the gap between fast-drying solvents and long-lasting film-formers. The compound stays stable under typical storage. Industry sees value in its compatibility with resins and pigments. In smaller workshops, I saw PGMEP help keep spray guns unclogged, all while limiting fumes that cause discomfort. This solvent remains an everyday choice for formulations where water makes things too slow and ketones cut too quickly.
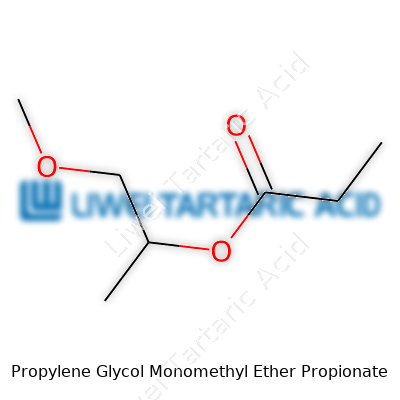
PGMEP brings a boiling point near 150°C, with a flash point above 42°C. Its moderate water solubility lets it mix with both polar and nonpolar compounds, which opens doors in paint laboratories. This ether propionate displays a mild, ether-like odor and flows well without high viscosity. The chemical formula, C7H14O3, points to its range of possible modifications. Density lands around 0.96 g/cm³. Over years of storage testing, I noticed its resistance to hydrolysis and autoxidation compared with earlier glycol ethers. Besides, the lack of strong reactivity with most container materials means storage hassles fade into the background.
Regulations demand that every drum or pail lists identity, net contents, batch info, and hazard statements. Product grades come from established standards like those from ASTM. Purity usually stays above 99%, with water content and color carefully controlled. In my factory visits, I learned that specifications on acidity and distillation ranges carry direct consequences for finished products. Handling guidance centers on fire safety and avoiding skin contact. Without proper technical documentation, coatings makers risk spoiled batches and downtime. Labels always spotlight its UN number and correct chemical names so that no one mistakes its properties.
Manufacturing typically involves reacting propylene oxide with methanol to generate the monomethyl ether, followed by esterification with propionic acid. In plant settings, workers charge reactors with catalysts, manage pressure and temperature, and continuously monitor product purity during separation steps. The final distillation strips unwanted water and light fractions. Waste minimization matters, so process engineers save energy by recycling unreacted feedstocks. Decades of optimization have improved selectivity, so less undesired byproduct ends up downstream.
PGMEP stands up to ester hydrolysis in basic or acidic conditions, generating propionic acid and the monomethyl ether of propylene glycol. Its functional groups allow for moderate reactivity. During copolymerizations or derivatization, this compound participates as a solvent or sometimes as a latent carrier of functional groups for blocking isocyanates or dispersing pigments. In early bench-scale trials, I observed how tweaks in formulation affected not just application but also chemical stability. Suppliers experiment with purity and side groups to meet specialty coating requirements.
Around industry, PGMEP carries various labels. Trade names like Dow’s DOWANOL PMA or Eastman’s PM Acetate get thrown around. Internationally, you’ll see “Propylene Glycol Methyl Ether Propionate,” “1-Methoxy-2-propanol acetate,” or simply “PMAc.” Clear identification prevents mix-ups, something I learned the hard way in a shared warehouse. On paperwork, the CAS number 143-32-2 provides a common reference. Consistency on naming matters for procurement, safety checks, and communicating within multi-lingual teams.
Fire and workplace safety always take priority. PGMEP earns a flammable label thanks to its flash point. Skin absorption remains moderate compared with earlier ethers but still calls for gloves and splash-proof goggles. In ventilation-challenged paint shops, exposure can trigger headaches or minor irritation. Regulatory bodies like OSHA in the US and ECHA in Europe set exposure limits and require safety data sheets on hand. As rules evolve, companies upgrade storage with explosion-proof fittings and invest in worker training. Reliable fume extraction and spill management form part of daily operations, not just annual audits.
The coatings sector leans heavily on PGMEP for waterborne and solvent-based resins. It keeps acrylic or polyurethane paints workable, enables crisp lines in printing inks, and ensures thorough dissolution of dyes. In electronics, PGMEP acts as a cleaning agent for circuit boards, thanks to its bright balance of solvency and evaporation rate. Wood stains and finishes take on better penetration with this solvent. While in customer-facing roles, I often answered questions from small-batch producers who prized its gentle touch with delicate pigments. Manufacturers value how PGMEP maintains surface smoothness in automotive repair and industrial coatings.
Ongoing research targets better toxicity understanding, sustainable production, and improved performance. Universities and corporate labs look for green process routes, minimizing hazardous byproducts. In recent years, computer models and pilot reactors helped chemists tweak catalysts and recycle streams, lowering greenhouse emissions. Safety research kicks up during regulatory updates, as more data pours in on chronic exposure and environmental impact. R&D also focuses on tailoring PGMEP blends for niche applications in electronics and aerospace. Engaging with innovator networks, I’ve seen partnerships sprout between chemical producers and coatings giants to pilot new solvent mixtures.
PGMEP carries a moderate toxicity profile. Inhalation or skin contact doesn’t hit as hard as older solvents like toluene or ethylene glycol ethers, but chronic exposure may affect liver enzymes or trigger mild respiratory issues. Animal studies from the past showed low acute toxicity and limited bioaccumulation. Modern labs continue to probe reproductive and long-term effects. Environmental scientists monitor for aquatic toxicity, since persistent discharges from manufacturing sites can affect local flora and fauna. Staying up-to-date on toxicity data means industries adapt PPE practices and report transparently to communities. From years spent with EHS teams, I saw that erring on the side of caution helped prevent both incidents and regulatory headaches.
Industry trends point to greener solvents, with PGMEP playing a role so long as process improvements keep emissions and energy use down. Regulatory scrutiny intensifies each year, pushing for lifecycle assessments and full traceability. Research into bio-based propylene glycol and cleaner esterification signals an emerging wave of sustainable innovation. Downstream, coatings formulators ask for better health and safety profiles, while electronics manufacturers seek solvents that leave less residue and degrade promptly. For producers, embracing transparent sourcing and open communication about hazards builds trust with end-users and regulators. PGMEP’s future rests on adaptability, robust supply chains, and continued investment in both worker safety and environmental stewardship.
Propylene glycol monomethyl ether propionate, known as PnP among chemists, pops up in more places than most folks realize. You don’t find it in the kitchen or medicine cabinet, but it quietly fuels a lot of industries that shape modern life. Talking with workers on the manufacturing floor, you hear the same thing: solvents like this one keep everything moving—literally and figuratively.
One of its big claims to fame rests in paints and coatings. Painting a car or your living room wall isn’t just about color and creativity. The chemicals beneath the surface decide whether paint stays smooth, dries on time, or peels after a few hot summers. PnP gives paint that perfect blend of fast drying with enough time to spread a clean layer. It does this without making the workplace smell like trouble, since it brings less harshness compared to older solvents.
Tape recorders and circuit board factories once smelled like old chemistry labs for a reason. Electronics manufacturing leans hard on solvents to clean circuit boards and prep components. In my experience visiting electronics plants, workers always mention safety. They prefer solvents that get the job done without rough fumes, and PnP delivers. You walk out of a facility that uses products like PnP without that pounding headache you sometimes get elsewhere.
Over in commercial printing, this solvent keeps print heads flowing, ink spreading nicely, and machines running well. No one wants blotchy magazines or streaky flyers. People in that world care about clarity and reliability. If the press jams, business stalls. Adding a well-balanced solvent like PnP helps keep production lines rolling.
Chemical safety deserves real talk, because regulations aren’t just paperwork—they protect real people. Companies want ingredients that score higher safety marks. PnP lands better than many alternatives in health studies, mainly because it breaks down more easily in the body and environment. Agencies keep a close eye, and so far the data shows that workplaces using PnP see healthier air, fewer headaches, and a lower risk of harm over time. I’ve met workers who say they notice the difference, especially at the end of an eight-hour shift.
Regulators and environmental advocates push for greener solvents every year. PnP isn’t perfect—no chemical is without risk—but it brings lower toxicity and less smog-forming vapors compared to some of the old standbys. In my time researching new green chemistry, I’ve seen PnP used in experiments aimed at cutting back on greenhouse gases and volatile compounds. Cleaner air in industrial neighborhoods isn’t a luxury—it’s about fairness and public health.
Some engineers turn to water-based options or even biobased solvents, but moving away from tried-and-true formulas can get expensive. Stronger research helps. More investment in safer chemistry means jobs get done with less risk. Companies smarten up by retraining staff, updating equipment, and listening to scientists who study long-term effects.
When people ask why a certain chemical matters, I remember what I’ve seen on factory floors and in labs. Keeping industry running safely doesn’t rest on one miracle ingredient, but smart choices like PnP push things forward. Every safer step, big or small, counts for workers, neighborhoods, and anyone breathing the air beyond the factory fence line.
Propylene Glycol Monomethyl Ether Propionate pops up a lot in everyday products. You find it in paints, inks, cleaning products, and even coatings for furniture. The industrial world values this solvent for its strong performance and its ability to dissolve tricky ingredients. When most people first hear the name, it sounds more intimidating than it needs to. But the real question is, does it pose a risk to our health or the environment?
I’ve worked in a few workshops and done my fair share of painting and refinishing. Products ranging from water-based paints to floor polishes mention Propylene Glycol Monomethyl Ether Propionate on the label. In practical terms, this chemical helps paints dry faster and streak less. It lets products adhere better, which saves money and effort. But like any strong solvent, it can affect you if you're not careful.
The US Environmental Protection Agency (EPA) and the European Chemicals Agency (ECHA) have both taken a close look at this ingredient. Toxicologists often point out that skin contact and inhalation are the main routes of exposure. In most cases, it’s not known to cause long-term harm if you use it in well-ventilated spaces and avoid direct contact. Repeated exposure, though, can cause headaches, dizziness, and mild skin irritation. A lot depends on the way you use the product—if you pour or spray large amounts inside a closed room, the risks go up.
People who paint indoors know about ventilation. I remember working one winter, the windows closed and heat blaring. I didn’t respect the warnings and noticed my throat and eyes stinging. After that, I got in the habit of fans and open windows. Gloves and goggles seem like overkill for quick jobs, but even careful folks can splash by accident. Getting a little careless with solvents means you might end the day with red knuckles and a headache.
Label information sometimes reads like legalese, yet it’s worth a minute. Manufacturers have to spell out potential hazards and safe handling. The instructions tell you if you should wear gloves, avoid sparks, or call for fresh air. Skipping these steps never ends well. The best lesson comes from the professionals—no one wants to lose workdays to preventable mistakes.
Companies making products with Propylene Glycol Monomethyl Ether Propionate do keep safety in mind. Major paint and chemical companies run frequent safety tests, follow rules on airborne concentrations, and share safety data sheets with details on exposure limits. OSHA puts workplace limits in the United States, so workers don’t breathe dangerous levels all day. These organizations regularly review new studies to make sure the rules reflect current science.
If you’re using anything that lists this solvent, a few habits go a long way. Good airflow and protective gear knock back the biggest risks. Never mix chemicals unless labels say you can, because reactions with other solvents might make things worse. Storage matters, too—keep containers closed and away from heat. If you spill any on your hands, wash right away instead of just wiping off.
Propylene Glycol Monomethyl Ether Propionate works well and remains in use for a reason, but like with a lot of other household and industrial chemicals, regular common-sense habits do the heavy lifting. Reading up before the job saves time in the end, whether you work with solvents every day or just once in a while.
Propylene glycol monomethyl ether propionate, often shortened to PGMEE, plays a major role in many coatings and inks. In places where people work with this solvent daily, safe storage and careful handling matter a lot. Over time, most chemical incidents have come not from large accidents, but from small mistakes in storage. Leaving a drum next to a heat source or in the wrong container can spell trouble quickly.
PGMEE is a flammable liquid. Laboratories and factories keep it away from open flames, sparking equipment, or even simple heat lamps. Fireproof storage cabinets rated for flammable materials keep risks in check. Proper ventilation in storage rooms keeps vapors from building up. Nobody wants a headache from fumes, and regular air checks help spot leaks early.
Staff check drum seals often. Even a hairline crack or loose cap can release enough vapor to raise alarms. Many places use explosion-proof refrigerators when low temperatures are required. In crowded warehouses, dedicated zones with secondary containment bins help avoid mix-ups with incompatible materials like acids or oxidizers. Clear labeling helps new team members steer clear of trouble.
Anyone who’s handled solvents can recall a time when they underestimated the amount that splashed or spilled. Chemical-resistant gloves and good eye protection save a lot of pain. Many workplaces require lab coats or coveralls and keep emergency showers close by. Even brief skin contact with liquid solvents should not go untreated. Washing hands after work, and before meals, stays standard practice.
Moving drums means more than just lifting. Workers train with drum trolleys to prevent strains and avoid accidental drops. Seals get checked before every move. Opening a container slowly, while pointing the vent away from your face, stops unexpected sprays. People new to the job often learn this lesson the hard way, so experienced staff usually model the right habits in front of them.
Spills happen, even in the best-kept shops. That’s why spill kits with absorbent pads and neutralizing agents stay close to every storage area. Teams practice for simulated incidents a few times each year, building muscle memory for how to catch drips or sweep away a puddle. Quick action on a small spill can keep production running and prevent project delays. Keeping the materials Safety Data Sheet close at hand avoids confusion in tense moments.
Getting storage and handling right takes regular review. Staff feedback often brings fresh ideas: wider aisles for moving drums, better airflow, or swapping out worn out personal protective gear. Leaders set aside budget for fresh containers and up-to-date signage. New hires watch training videos, but, more importantly, they learn by shadowing workers with years of experience. Knowledge travels best in direct, practical ways.
No shortcut replaces vigilance. Caring for chemicals starts with simple routines that add up, day by day. Safer storage and handling of PGMEE means smoother operations and fewer worries for everyone working with it.
Propylene Glycol Monomethyl Ether Propionate, often called PnP or PGMEP, plays a quiet but critical role in a huge range of products. You find it behind the scenes in paints, coatings, inks, and cleaning agents. My first brush with it happened in an automotive shop where I worked as an assistant. We would use water-based paints, and PnP was always on the ingredient list. It never stood out—just a colorless liquid with almost no odor—but it got the job done, blending well with other chemicals and keeping things smooth.
PnP is a clear liquid. It slips out of a bottle looking a lot like water and has a faint, almost sweet smell. It evaporates slowly compared to acetone but faster than plain water. With a boiling point near 146°C (295°F) and a melting point well below freezing, this solvent performs well in both chilly and warm environments. That made it useful in my shop—paint jobs and cleanups just didn’t slow down, even when winter hit.
It mixes easily with common solvents and, to some extent, with water. This makes cleanup and blending more manageable. Its moderate vapor pressure means it won’t vaporize like crazy and fill the room, but you still want good ventilation. I remember a time someone forgot about that, and the faint odor turned into a headache for a few workers by lunchtime.
PnP stems from the ether family, combined with an ester backbone. Chemists designed it to balance solvency and safety. Its ether-ester combo means it unlocks grease, dirt, and stubborn residues, which is why paint strippers and cleaners keep it around. From a safety standpoint, its flashpoint sits pretty high (around 47°C or 116°F), so it’s less likely to ignite than many older solvents. Years ago, we used more flammable chemicals, and there was always tension near the shop heaters. PnP changed things for the safer.
PnP doesn’t corrode metal fast, and plastics tend to survive a soak. That compatibility reduces replacement costs for tools and tanks. The downside? Extended exposure might cause some rubber seals to swell. Keeping spare gaskets made up for that, but it’s something to look out for.
PnP isn’t completely without risk. Prolonged skin contact dries you out or, occasionally, causes irritation. A few of my colleagues would get red spots if they skipped gloves. Breathing in its vapor over long periods brings on headaches or drowsiness. Local safety rules treat it with respect, demanding extraction fans and good labeling. Though it’s much safer than the chemicals our parents used in shops decades ago, you still shouldn’t go easy on personal protective equipment.
Environmentally, PnP outperforms many older solvents. It breaks down in the air and water much more quickly and doesn’t stick around to pollute streams or soil like the chlorinated solvents from the past. I take this seriously—our family farm depended on clean groundwater, and chemical spills near the shop carried real stakes. Using a solvent like PnP, which is less persistent and less toxic to fish, just seemed like the right call.
The biggest challenge now comes down to balancing utility and impact. As industry regulations tighten and eco-labels become common, companies hunt for safer, greener solvents. PnP stands out, but nobody should treat it as harmless. Further innovation might deliver alternatives with even lower toxicity and more environmental advantages. Shops, factories, and even art studios benefit from knowing what’s in their products and asking for better options. I keep an eye on product sheets, and so should anyone who values their health, or the water bubbling beneath their town.
Propylene glycol monomethyl ether propionate, known to some as PGMEP or as a solvent in paints, inks, and cleaning products, calls for special care once a container runs empty. I've seen too many folks pour leftover solvents down the drain hoping they vanish without any consequence. That’s not just risky, it's illegal in most places and can seriously mess with local water treatment systems and the environment.
Water treatment plants struggle to remove trace chemicals, especially potent organic solvents like this one. Pumping them into sewers can lead to toxic build-up in rivers and lakes. Wildlife faces real harm because aquatic systems simply aren’t built to filter out human-made chemicals.
A couple of years ago at my workplace, we used PGMEP during a project overhaul. None of us wanted to shortcut disposal, mostly because we’d learned the hard way with a minor spill in the past — the odor alone made the office unlivable for a day. So, we followed the Material Safety Data Sheet (MSDS). The directions guided us to seal containers tightly and send them to a hazardous waste facility, not the local dump or down the sink. Extra time and expense felt worth it after talking with a neighbor whose well water picked up a chemical taste—traceable to improper waste handling by a factory up the road.
State and federal agencies like the EPA place solvents such as PGMEP in a regulated zone. These laws exist to protect both sanitation workers and the wider public from fumes, fire risk, and water pollution. Ever check your county's rules on hazardous waste? Most offer special drop-off points or collection days. Folks who choose to ignore these guidelines risk stiff fines, but more critically, leave their neighbors breathing and drinking what comes of those choices.
So what really works? My routine now includes labeling every used solvent container and sticking it away from living areas in the garage. Each spring, I take the batch to the local hazardous waste round-up — usually a Saturday affair where the county teams up with a disposal company. Staff know exactly how to deal with everything as soon as I hand it over. If you’re ever unsure, check the label and call the collection site for advice.
For workplaces with larger quantities, partnering with a certified chemical disposal contractor pays off. Contractors safely transport containers and issue real documentation that everything’s handled above board. In tight-budget operations, proper training keeps everyone safe and prepares folks for inspections.
Limiting bulk purchases goes a long way—most places don’t need gallons of strong solvent sitting around just in case. Buying right-sized containers and planning jobs to use up full amounts prevents headache later. And there are safer options: water-based products are catching up in performance for more jobs, and swapping for less toxic formulas, when feasible, keeps storage and disposal simpler.
The bottom line lands on each of us. Safe disposal of chemicals like propylene glycol monomethyl ether propionate isn’t just following rules, it’s about showing respect for neighbors and future generations by treating our shared resources as something worth protecting.