Propylene Glycol Methyl Ether, better known in laboratories and industry as PGME, didn’t jump onto the scene all at once. It took steady progress through the 20th century, spurred by the drive for better, more versatile solvents. Chemical companies pursued alternatives to ethylene-based glycols when environmental and safety regulations grew tighter in Europe and North America during the 1970s and 1980s. Those years brought a wave of investments in catalytic processes using propylene oxide, and PGME stepped up as one solvent that found its way into almost every corner of industry—from paints and cleaners to electronics. Regulatory milestones like the Clean Air Act in the United States kept shaping how companies approached glycol ethers, helping PGME secure its spot by balancing solubility with lower toxicity compared to some early solvents.
Anyone working with PGME for a few years can tell the value of a solvent that cuts through grease, paint residues, and printing inks without causing absolute havoc for skin or lungs, given proper handling. Its gentle odor, moderate evaporation rate, and just-right mix of water and oil solubility give workers flexibility across jobs. Chemical plants use both technical and high-purity grades, depending on the end use. Lower-purity grades fit the bill for paints, cleaning formulations, or general degreasing, while electronics manufacturers or pharmaceutical labs look for top-grade solvent with tighter controls over aldehydes and water content. Every drum or tank comes with the markings, but a hot summer warehouse or a poorly vented drum can remind you fast that storage and handling need close attention despite its friendly name.
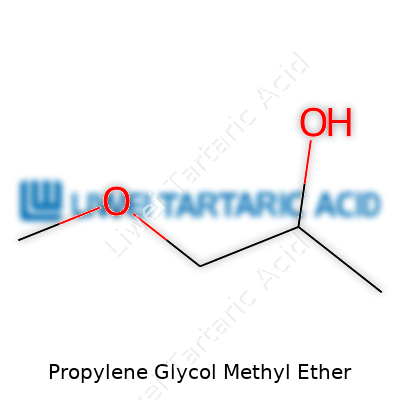
PGME stands out in the glycol ether family because of its molecular structure. With a formula of C4H10O2, it boils at about 120°C, which puts it just above water but well below some heavier solvents. Its vapor pressure keeps things manageable in terms of evaporation, so large surface spills don’t disappear in minutes or choke up a workspace. On your gloves or workbench, the feel is slick but not greasy. Its miscibility lets you mix it with water, alcohols, and most organic chemicals, so it bridges the gap when you need a single solvent to work in a complicated blend. Flammability does show up as a concern, with a flash point around 31°C, so fire prevention deserves attention wherever PGME is in play.
Barrels and containers bearing the PGME name follow guidelines from organizations like ASTM and local chemical agencies. Labels always note critical stats—purity minimums (usually above 99% for technical grades), water levels, and key impurities. Shipping papers carry standardized identifiers like UN 1993 and hazard classes, warning handlers about flammability and proper venting rules. Safety Data Sheets lay out proper storage temperatures, spill response, and first aid for exposure. Chemical suppliers include batch-specific test results and traceability details, so if a problem pops up in production, tracking it back doesn’t turn into mystery work.
Large-scale production of PGME happens through the reaction of propylene oxide with methanol. Factories use strong acid or base catalysts to keep the reaction efficient, tuning the temperature and pressure while cycling the reactants through columns for separation. After synthesis, plants follow up with distillation steps—sometimes two or three—to meet tight purity specs, skimming off lower- and higher-boiling byproducts. Every step comes with its own quirks; too much heat or a slip in catalyst concentration can mean methyl ethers cross-react, cutting into yield and cranking up overhead costs.
PGME reacts most easily at its ether group, making it a popular starting material for other solvents or specialty chemicals. In labs, chemists sometimes use strong acids to break it down or modify it into longer-chain glycol ethers. Its methyl ether group resists oxidation under regular storage, unlike some aliphatic alcohols that spoil or grow acidic in half a year. Beyond that, with decent control, PGME serves as a reactant for surfactant production, particularly for non-ionic detergents. Mixed with strong acids or bases in synthetics labs, PGME takes part in transetherification or esterification reactions, all driven by industries looking for new cleaning or performance fluids.
Across catalogs, PGME shows up under names like 1-Methoxy-2-propanol, Propylene glycol monomethyl ether, or just Dowanol PM in certain markets. Different language regions or suppliers use their codes—PM solvent or Propasol P aside—but the underlying material remains the same. Some older firms even label it as PGME-P for “primary” to set it apart from secondary isomers, since isomer mix-ups can cause performance headaches in coatings or electronics under odd conditions.
Plants and storage rooms set up with PGME stick to OSHA guidelines, keeping fire extinguishers and electrical equipment rated for flammables right where they’re needed. Workers wear gloves and goggles because prolonged skin contact can stir up irritation, even if the solvent doesn’t “burn” on contact like some. Good ventilation plays a huge role, especially if heating tanks or open pouring runs through a shift. Leaks get handled using absorbent materials, collected quickly and disposed of as hazardous waste, following both EPA regulations and local landfill guidelines. Routine monitoring—like sniff tests backed with electronic detectors—cuts down on surprise vapor buildup. Long-term workers remember the musty but sweet smell, training their noses as much as any digital monitor.
PGME carries its weight in several industries. Paint and coating manufacturers rely on its ability to dissolve resins and speed up drying times without wrecking performance. Print shops use it in ink formulations, loving its balance for quick transfer and thorough cleanup afterward. Electronics plants bring it into the picture for circuit-board cleaning, where its volatility and low residue mean tiny contact points stay clear of gums or deposits. Industrial and institutional cleaners count on its grease-busting power when blending strong-yet-manageable cleaning solutions. In automotive and aerospace, it slips into degreasers and prepping agents before assembly and painting. Even laboratories keep a bottle handy to clear sticky residues or prep samples for analysis.
Chemical companies and material scientists keep searching for new pathways to produce PGME with fewer emissions and better energy efficiency, given pressure from green chemistry goals and carbon tax rules. Trials run on catalysts that skip heavy metals, aiming for lower waste and longer service life. Some research teams tinker with bio-based propylene oxide feeds, targeting routes that let plants or agricultural waste substitute for fossil fuels. PGME’s value as a solvent means coatings experts test blends with fluoropolymers or nano-fillers, seeing how it tweaks performance in extreme environments. Pharmaceutical labs watch for low-toxicity alternatives, hoping that PGME derivatives can carry actives deeper into formulations without sparking allergic reactions.
Scientists and regulators have spent years mapping out the safety profile for PGME. Inhalation studies with lab animals point to reversible respiratory irritation at high concentrations, much like other small glycol ethers. Skin studies highlight irritation risk with repeat exposure, but PGME doesn’t show the reproductive impacts that have haunted some related compounds. Epidemiological surveys in manufacturing settings track exposure levels, often coming in well below occupational limits set by ACGIH or government bodies. Medical examiners look out for headaches or dizziness in over-exposed workers and recommend improved ventilation or respiratory protection for big jobs. Wastewater monitoring adds another check, making sure careless spills or drains-out don’t tip up aquatic toxicity levels in local water bodies. Compared with many solvents used a generation ago, PGME stands out for lower risk but never gives a free pass on safety.
Industry-wide shifts toward safer, lower-impact solvents keep PGME in the spotlight. Regulations tightening worldwide on VOC emissions and toxicity ratings give an edge to solvents with established safety records and lots of published data to back up claims. Chemical engineers work to upgrade process controls, squeezing down waste and energy use along every step from synthesis to shipping. Interest grows in bio-derived raw materials, since consumer and regulatory pressure don’t seem to be letting up. Research teams focus on improved formulas for fast-drying, high-gloss paints, or smarter cleaners for sensitive electronics, betting on PGME’s adaptability. New applications will probably show up in emerging tech fields—think batteries, flexible electronics, and sustainable packaging—where manufacturers demand both performance and a track record on safety. The lesson learned from decades around industrial solvents rings as clear as ever: stay nimble, keep learning, and build trust through transparency and shared knowledge.
Walk through any hardware store and you’ll find bright cans of paint, strong-smelling cleaning solutions, and stacks of adhesives. Behind the scenes, there’s a common thread among many of these products: propylene glycol methyl ether, often called PM. Folks in the industry use it every day, but it rarely draws much attention.
In the world of coatings and paints, consistency makes all the difference. PM acts as a solvent in water-based paints, thinning the mixture enough for it to roll on smoothly, without changing the finish or color. For DIY painters or professionals, this turns a frustrating chore into a job well-done. PM stands out among other chemicals because it helps paints dry at just the right speed—fast enough for convenience, slow enough to keep the surface even and streak-free.
Spend time working in hospitals, offices, or factories and you see how much rides on good cleaning solutions. PM lands in many of these formulas due to its ability to dissolve oils, greases, and stubborn stains without leaving heavy odors behind. Since it works well mixed with water, industrial cleaning teams choose it for both effectiveness and safety.
Remember the last time you picked up a brochure with crisp, colorful photos? Printers rely on PM to keep inks flowing. It keeps ink at the right consistency for high-speed presses and prevents clogs that waste both ink and paper. Choosing the right solvent can mean fewer jams and less downtime—a big deal in a busy print shop.
You’ll also find PM in everyday products like markers, cosmetics, and some perfumes. In markers, it stops felt tips from drying out before their time. Cosmetic manufacturers use PM because it helps blend ingredients in lotions and perfumes, making them easier to apply and more pleasant to use.
Like any chemical used around people, PM calls for clear rules and honest labeling. Too much exposure can cause health issues, such as headaches or skin irritation, and larger spills could pose environmental risks. Companies owe it to workers and consumers to test thoroughly and provide protective gear in big operations. The Environmental Protection Agency tracks the use of PM, pushing for best practices and regular reviews. A well-run plant makes safety part of the daily routine, not just a line in the rulebook.
As new products come out and regulations grow, some manufacturers look for greener options. Substitutes don’t always match PM’s performance, yet demand for less toxic chemicals keeps rising. By investing in research, the industry can keep people safe without losing the practical benefits that PM brings to the table.
Years spent working in chemical plants taught me that progress relies on knowing the materials you handle. PM may never become a household name, but a careful look behind the label proves its value in making life a little safer, cleaner, and more colorful.
Propylene glycol methyl ether shows up in lots of places: paints, cleaning solutions, printing inks, and cosmetics. For years, manufacturers have relied on it because it dissolves slickly in water and mixes well with other chemicals. Many people walk past store shelves loaded with products containing it and never realize they're letting this substance into their homes.
It’s important to look past obscure chemical names and see how safety really plays out. Nobody wants a surprise allergy or a health scare down the road. In industrial settings, workers face higher exposure. Home users get much lower doses, but the chemical still evaporates into the air and touches skin. I’ve worked with household cleaners myself. I usually check labels, and if strong solvents show up, I open a window and use gloves, just to be sure. One can never predict a skin rash or headache until it happens.
Let’s put confidence where it belongs: facts. The European Chemicals Agency and the US Environmental Protection Agency have both taken a good look at propylene glycol methyl ether. At normal exposure levels—like using recommended amounts of paint or cleaning sprays in a ventilated room—they say the substance doesn’t seem to cause birth defects, cancer, or major long-term health damage. But no chemical is completely risk-free. High concentrations can irritate the eyes, nose, and lungs. Skin might itch or dry out after long contact. If someone gets reckless and mixes several products together, vapors can add up fast and trigger dizziness or worse.
Science builds caution into any safety guideline. The danger comes from doing things outside the instructions. Pouring a bucket of it over a floor with no airflow? That’s a bad idea. I once used a strong solvent on an art project and learned quickly how overpowering a closed room gets, even after a few minutes. My nose burned, and I realized cracking a window wasn’t optional.
The safest route always starts with following usage instructions, wearing gloves, and keeping rooms aired out. Store products out of reach if kids or pets might get curious. Spills need quick cleanup. Wearing a mask might sound overboard, but I’ve grabbed one when sanding paint indoors—better safe than sorry.
Every batch poured down a drain ends up somewhere. Sewers and treatment plants can only handle so much. Propylene glycol methyl ether breaks down, but large amounts still stress local water. Communities are asking manufacturers to craft lower-impact products and offer full ingredient lists. As someone who’s moved several times, I’ve seen differences in air quality between old apartments where neighbors painted with open buckets and those with new eco-label products. It changes how people feel and how children play indoors.
Choices matter. I look for labels that spell out ingredients and provide steps for safe use. Companies and scientists can push for clearer, easier instructions. Alternatives exist for many solvents, though price points and performance still drive business decisions. More information helps everyone reduce risks and live healthier, whether at home or on the job. A little caution, some common sense, and better industry transparency go a long way in keeping everyone safe.
Propylene Glycol Methyl Ether turns up often in paints, cleaners, inks, and electronics. At first glance, it seems like just another clear liquid in the supply room, but this chemical can cause headaches—literally and figuratively—if ignored. It evaporates quickly and can easily slip into the air, leading to irritation or a fire risk. The flash point sits just below 90°C, so high temperatures bring real danger. Stash bulk drums in a shady, well-ventilated area away from process heat or open flames. Direct sun turns up the heat quickly in storage tanks, so awnings or cool, built-in sheds make sense. Metal containers rated for flammable liquids give the best protection against accidental leaks and sparks.
People handle chemicals every workday, and not everyone reads safety sheets before starting a shift. No surprise, a big part of safe storage comes down to predictable routines. Standard operating procedures can sound boring, but they work. Keep spill kits and fire extinguishers nearby—these aren’t decorations, but tools staff need quickly when things go wrong. Loose lids or open-fill pipes can invite trouble, so workers must double check connections. Local exhaust fans or open doors help keep vapors from building up around drums or mixing stations.
A simple label on every drum marks out what’s inside and cuts down on mix-ups. Manufacturers and old-school shop managers know that proper labels—ones that don’t smudge or peel—keep people safer. Segregation counts, too. Propylene Glycol Methyl Ether shouldn’t bunk up with oxidizers, acids, alkalis, or concentrated chlorine products. Even a small splash between incompatible chemicals can kick off a dangerous reaction. Most warehouses use painted floor stripes and dedicated sections to keep products apart.
Drums and totes need careful loading. Strapping containers during shipment isn’t just a formality. Rolling drums hitting each other cause dents or leaks. Drivers have tough jobs and sometimes deal with sloshing containers, sharp stops, or extreme heat in trucks. Farms, workshops, and distribution centers all face the same challenge: one sharp movement throws a poorly secured drum off balance, and then you’re cleaning up in the middle of a delivery route. For short hauls, it pays to check for leaks or damage before and after each trip.
Practical safety always wins over expensive gadgets nobody uses. Workers who handle the liquid need splash goggles and chemical-resistant gloves. A splash on skin stings, and the vapors can burn the eyes. Bigger operations might install eyewash stations and emergency showers. Good practice across industries means everyone washes up after handling the solvent, never eats or drinks around open containers, and keeps work clothes out of the breakroom. Company experience shows that regular drills on spill cleanup get everyone thinking ahead—seconds matter during an emergency.
Incidents with Propylene Glycol Methyl Ether usually come down to constant hustle and small oversights. Consistent training, modern storage solutions, and open communication between shifts all help. Supply chain managers who keep a close eye on temperature, labeling, and segregation rarely face big spills or worker health complaints. It’s not about fancy technology; it’s about real people taking each detail seriously.
People come across Propylene Glycol Methyl Ether in many workplaces, especially in paints, coatings, inks, and cleaning products. It’s easy to overlook what these chemicals can do if careful handling isn’t practiced. Breathing in its vapors can irritate the respiratory tract. Folks may start coughing, or feel a burning sensation in the nose and throat. Strong concentrations in enclosed spaces raise the risk, especially for anyone working long hours without proper ventilation.
Skin contact seems harmless at first, but repeated or prolonged exposure dries out the skin. Redness, itching, and chapping often follow. Some people even develop allergic reactions, a fact often reported among painters and cleaning staff. Splashes in the eyes sting, and vision blurs for a while. I remember a colleague who, despite wearing gloves, dealt with peeling skin from using chemical solvents daily.
Swallowing Propylene Glycol Methyl Ether by accident can upset the stomach, cause nausea, or make someone throw up. More serious symptoms—like headache, dizziness, and confusion—show up if a decent amount gets into the system. Long exposure sometimes leads to more serious nervous system effects. There are hospital reports of folks passing out after drinking from mislabeled bottles, which highlights why proper storage matters so much.
This solvent catches fire easily. Spilled liquid or vapors floating in the air can ignite from something as routine as a static discharge. Many industrial fires have traced their origins to places where chemical containers were left open or transfers happened near pilot lights. Folks working in auto shops or printing presses usually double-check for leaks and static before opening a drum for this reason.
Once, I witnessed a spill near a storm drain. Even small amounts that reach waterways can harm aquatic life. The compound breaks down slowly in the environment, and toxic stress can affect fish and invertebrates. Urban water systems often struggle with traces of solvents, making regular accidental releases a community problem, not just an industry hiccup.
Good ventilation changes everything. Shops with big fans and open doors keep vapor levels down. Workers benefit from well-fitting gloves, goggles, and proper respirators. Regular training on safe chemical handling, along with easy-to-read labels, avoids a lot of trouble. In my experience, folks skip steps when short on time—so routines and checklists help keep up good habits. Safe storage—away from heat or flame—cuts down on accidents, as does using closed transfer systems for liquids.
Simple habits lower the odds of problems: washing hands before eating, not mixing work clothes with regular laundry, and checking safety data sheets before starting a job. When companies also invest in spill kits and teach people how to use them, cleanup becomes safer and faster. At the end of the day, chemical solvents come with risks, but steady attention to protection and solid workplace practices keeps people and the environment safer.
Anyone who’s spent time in a lab or a coating shop knows that solvents can make or break a formula. Propylene glycol methyl ether stands out for its strong reputation as a “universal” middleweight. Experience shows that people reach for it because it bridges the gap between water and stronger, less forgiving solvents. It mixes smoothly with water—there’s no stubborn layering or clumping, which is a headache with others. Engineers working on water-based paints, for example, look for that trait since it lets them blend in other ingredients with fewer problems.
You pour propylene glycol methyl ether into water and watch them blend without fuss. That’s not just convenient—there’s chemical magic in how this molecule plays nicely with polar and non-polar compounds. In settings like cleaning products or coatings, this means more flexibility. If you’ve struggled to get alcohols or glycols and their water content to combine, adding this agent can save the day.
Beyond water, this glycol ether also gets along with organic solvents like ethanol, acetone, and even tougher customers like toluene. Industrial paint and ink manufacturers often take advantage of this, building solvents blends that can be adjusted depending on drying speed or cleaning needs. These folks value predictability, and this chemical offers that, which counts for a lot under pressure.
Safety officers see propylene glycol methyl ether as a tamer choice compared to older solvents such as toluene or xylene. It evaporates more slowly, lowering inhalation exposure and risk of headaches. The U.S. Environmental Protection Agency recognizes it as less toxic and less likely to linger as environmental residue. Still, gloves and eye protection matter, because even less aggressive solvents can dry skin or cause irritation when splashed. In shops where kids or pets might pick up a dropped rag, safety gains real meaning.
Regulatory requirements have tightened on emissions, especially in regions like California, setting limits on volatile organic compounds (VOCs). Switching to this solvent can help companies meet those standards without sending costs out of control. It has allowed formulators to avoid the disruption of reworking entire product lines, as seen in cleaning and ink manufacturing. All of this doesn’t happen by accident—teams check new blends in both lab and field, relying on clear protocols and experience.
Still, every solvent comes with trade-offs. Propylene glycol methyl ether burns at a lower temperature than water, so storage keeps fire safety front and center. Spill cleanups demand attention, as this liquid can sneak through porous concrete or disappear before you notice. Businesses often add secondary containment and educate staff about odor and ventilation. Budgeting a bit more on personal protective equipment and regular training brings down risk.
Disposal shouldn’t be ignored, either. Local regulations shape how facilities collect and treat wastewater containing glycol ethers. Some sites invest in on-site treatment or contract specialists to handle disposal, which keeps them clean and avoids fees. More research into biodegradable options or recovery processes offers hope for shrinking the environmental footprint long-term.
Propylene glycol methyl ether delivers a sweet spot for flexibility, safety, and cost. Whether in a lab, workshop, or production setting, it supports innovation while still answering calls for responsible handling. People don’t always talk about the science behind a solvent, but in the right hands, it opens up cleaner and smarter ways to get the job done.