Long before the chemical shelves grew crowded with complex synthetics, Propylene Glycol Diacetate started to gain attention from industrial chemists and product formulators. Its roots trace back to the post-war period, when the chemical industry pushed for safer, more versatile alternatives to strong solvents like toluene and xylene. These older chemicals had high vapor pressures and extreme toxicity, fueling a search for something that could deliver solvency without the baggage. Propylene Glycol Diacetate, crafted from propylene glycol and acetic acid, moved into view. Over the decades, producers in North America, Europe, and Asia have scaled up its manufacture, building on advances in reaction control and purification. Every step forward has helped move this solvent from obscure labs into paints, coatings, inks—and plenty of daily-use products.
Propylene Glycol Diacetate doesn’t shout for attention on ingredient lists, but anybody who’s ever painted a wall, used a permanent marker, or cleaned up acrylic glue has likely benefitted from its properties. Chemists turn to this ester because of its medium evaporation rate and balance of hydrophilicity and lipophilicity—it blends well with both water and organic solvents. Its clear, nearly odorless liquid form sets it apart from punchier, nose-burning alternatives. Producers often market it under a handful of aliases, and demand has steadily climbed as regulations crimp use of more hazardous options.
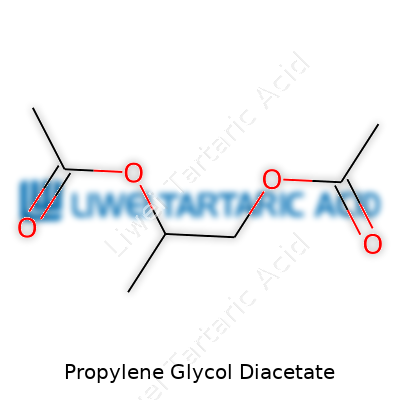
In the warehouse or in the lab, Propylene Glycol Diacetate comes off as a clear, colorless liquid. The molecular formula, C7H12O4, fits easily into the world of esters. Its boiling point sits around 240°C, while the flashpoint—important for handling in large volumes—hovers near 102°C. That brings a decent window of safety compared to lower-boiling acetates. Its density, close to 1.07 g/mL, means it neither floats nor sinks away from standard organics. Solubility tests show it will dissolve well in alcohol, ether, most standard organics, but not so much in water, giving it real utility in complex solvent blends. In terms of reactivity, it stays stable against oxidation at ambient conditions, rarely breaking down until pushed by strong acids or bases.
Producers ship Propylene Glycol Diacetate to manufacturers and labs with key information: purity above 98%, water content below 0.5%, and free acid content typically lower than 0.05%. Labels highlight the correct UN number for transport safety, along with hazard warnings aligned with GHS classifications. In my work, I have seen how crucial traceability codes have gotten—every barrel now houses batch information for downstream tracking. Customers request specific analysis sheets to ensure compliance with restrictions for VOCs and toxicity, reflecting how regulations never seem to loosen in this sector.
The standard pathway to Propylene Glycol Diacetate starts with a reaction between propylene glycol and acetic acid (or its anhydride), usually over an acid catalyst like sulfuric acid or a resin. The trick sits in controlling the temperature and removing water as it forms, because water limits yield by driving the reaction backward. I’ve watched operators tune vacuum and heat in pursuit of a dry, clean ester layer—distillation follows for final purification, leaving the signature neutral odor and clear finish. Equipment cleanliness, careful catalyst handling, and constant monitoring help prevent byproduct contamination.
Propylene Glycol Diacetate resists casual breakdown except under tough acidic or basic conditions. Under hydrolysis, acids or alkalis will split it back to its parent glycol and acetic acid, which sometimes comes into play in waste treatment or recycling operations. On the modification front, researchers chase new derivatives for specialty coatings or functional fluids by swapping acyl groups or tacking on side chains. These kinds of changes can shift volatility, solvency, or biocompatibility, always in response to new regulatory pressure or customer need.
Anyone shopping for this chemical may find a surprising array of names: PGDA, 1,2-Propanediol diacetate, Propanediol diacetate, and the lesser-used 2,2'-Dihydroxypropane diacetate. Some suppliers, mainly from Europe or Asia, slap on legacy trade names. This landscape of synonyms often creates confusion, so cross-checking CAS numbers becomes a survival skill in both lab and warehouse procurement. Reliable suppliers keep detailed SDS (Safety Data Sheets) handy, a necessity as product audits ramp up across industries.
Operators working with Propylene Glycol Diacetate follow evolving guidelines, mostly focused on fire risk (since it rates as combustible) and safe ventilation (to avoid buildup of vapors). Spills tend to be manageable, with established methods for cleanup using absorbent material. Eye and skin contact rules require gloves and goggles, although its relatively low toxicity compared to old-school solvents reduces fear on the shop floor. Transport by road or rail must conform to hazardous materials regulations, and secure drum handling training remains part of every site’s safety culture.
Propylene Glycol Diacetate finds steady use as a coalescing agent in waterborne paints, helping particles come together smoothly. Packaging inks, adhesives, and acrylic coatings rely on it for its evaporation profile and solvency. Formulators appreciate how it softens or dissolves certain resins that water or alcohol alone would struggle with. In cleaning and household products, its mildness and low odor win points where consumer complaints matter. Green chemistry efforts look at it as a candidate to replace more problematic solvents, and compliance with VOC regulations keeps it in the running for reformulated consumer goods.
Chemical companies and university groups consistently run tests to expand Propylene Glycol Diacetate’s role in next-generation coatings or specialty printing. Some teams chase lower-VOC blends for markets in California or the EU, while others probe for uses as a carrier fluid in agriculture or pharmaceuticals. Research teams hungrily assess the molecular skill set—looking to optimize performance by tweaking blend ratios, or by pairing with certain resins or surfactants. Collaboration with regulatory bodies often pushes the field toward cleaner, greener synthesis techniques, casting a constant eye on downstream safety and sustainability.
In terms of toxicity, Propylene Glycol Diacetate lands in a safer bracket than many older industrial solvents. Acute toxicity studies, both in vivo and in vitro, show relatively low risk for skin and eye irritation. Inhalation at high concentrations causes discomfort, but not the severe injuries linked with many aromatic solvents. Environmental impact assessments flag its ready biodegradability; wastewater streams containing the compound don’t present the sort of alarm that haunts persistent organics. Toxicologists remain vigilant, keeping an eye on possible chronic effects. Long-range studies help clarify if cumulative exposure links to endocrine or reproductive disruption—a concern always worth attention, given tightening safety standards globally.
Industry watchers predict growth for Propylene Glycol Diacetate as companies move away from high-VOC, high-toxicity options. Many paint brands already load it into their low-odor, green-labeled products. Emerging fields like printed electronics and specialty polymer manufacturing test it for performance tweaks, especially as bio-based raw materials become more attractive. Green chemistry advances may yield new, renewable synthesis routes that further boost its credentials. Every successful application lays groundwork for future research, and as regulatory limits tighten everywhere from California to Shenzhen, demand for safer, cleaner solvents is set to climb.
Most people walk past propylene glycol diacetate every day without knowing it. It's not the sort of thing splashed on labels at the grocery store. The truth is, this clear liquid sneaks into plenty of spots where clean scents and streak-free finishes matter. Its job reaches farther than most realize.
Anyone who has painted a wall or wiped down a counter probably got a first-hand look at what propylene glycol diacetate can do. It steps in as a solvent for both latex and oil-based paints. Thick globs dissolve, and colors spread more evenly because of this ingredient. Sometimes paint dries too quick or turns tacky. Additives like propylene glycol diacetate even out the pace, giving surfaces a smoother look and fewer streaks.
Cleaning products take a similar route. Grease, polish, or soap scum can cling in the kitchen or bathroom. Here, propylene glycol diacetate softens the grime and helps break it up without a harsh chemical smell or oily film left behind. Its ability to dissolve both water-loving and oil-loving messes sets it apart from most other common cleaning solvents. The EPA keeps tabs on its safety, and it's listed in the Safer Choice Ingredient List, which signals a lower risk profile than harsh industrial cleaners.
In fragrance work, something needs to carry those powerful scents without changing their smell or leaving a bitter note. Propylene glycol diacetate gives perfumers and manufacturers a hand. It carries fruity, floral, and soapy fragrances and helps them hold together. Sprays, plug-ins, and gels last a bit longer on the shelf or in the air because of it.
Cosmetic companies use it when blending makeup, lotions, and creams. Thick creams smooth out more easily, and pigments blend without clumping. The compound's low odor keeps products from smelling off-putting. Dermatologists rarely see allergic reactions from it, which matters for makeup that sits on skin for hours.
Propylene glycol diacetate breaks down in nature faster than many petrochemical solvents. According to data from the European Chemicals Agency, it doesn't build up in plants or animals. But that doesn’t mean it's free of all risks. Overexposure or improper handling can cause skin and eye irritation. Workers handling drums at factories need gloves and goggles—nobody wants it splashing in their eyes.
Plenty of companies face pressure to shift away from ingredients tied to pollution and health risks. Propylene glycol diacetate checks some important boxes: lower odor, moderate toxicity, and better biodegradability. Still, research should continue into even safer alternatives. Smaller manufacturers might not know that safer options are out there, so ingredient transparency remains a smart strategy. If companies share more about the solvents they use, people have a chance to pick products aligning with their values and comfort level.
In my own home, I look for cleaning products rated by Safer Choice or EWG Verified. More brands front-load ingredients like propylene glycol diacetate for good reason: They strike a balance between performance and risk. Shoppers, builders, and cleaning staff get a little peace of mind, knowing the ingredient does its job—without too much drama.
Open up any modern moisturizer, makeup, or shaving cream, and the ingredient list reads like a chemistry textbook. Propylene Glycol Diacetate pops up more often these days, raising questions from shoppers who check labels and wonder about the safety of what touches their skin.
Manufacturers turn to Propylene Glycol Diacetate because it dissolves other ingredients well and boosts the texture. This clear liquid adds a lightweight, non-sticky feel that fits many formulas, from fancy foundation to off-the-shelf sunscreens. Some chemists use it to keep oils from separating so lotions glide on smoothly. But the name alone tends to spook folks.
Regulators have tackled this ingredient for years. In the United States, the Food and Drug Administration (FDA) allows its use in food (as a flavoring agent) and in cosmetics. The Cosmetic Ingredient Review (CIR) expert panel checked out the toxicology data, which includes skin irritation and absorption studies, and marked it as safe at typical concentrations.
Research shows the body breaks down Propylene Glycol Diacetate quickly after application. Animal and human tests rarely turn up allergic reactions or signs it soaks deep enough to raise red flags. The amounts used in beauty products—around 1-5%—stay far below levels that cause problems in lab settings.
The bad reputation comes mostly from confusion. It shares chemical relatives with common solvents and antifreeze, which sends up warnings in blogs and posts. But pure Propylene Glycol Diacetate made for cosmetics gets highly purified and doesn’t share the risks seen in other chemicals in its family. Reports of mild irritation pop up, especially with damaged skin or high concentrations, though this happens rarely.
Allergists rarely list it as a common trigger for rashes. Routine patch tests look at thousands of patients, and very few react to Propylene Glycol Diacetate. Still, no ingredient can claim to be universally harmless. Some people react to water or fragrance just as easily.
Shoppers want straight answers about what goes into each bottle. Dermatologists and toxicologists keep up with new studies, giving updated advice when new risks or benefits pop up. Industry groups publish guidelines about concentration limits so any risks stay low. For people with sensitive skin, patch testing new products before applying them all over helps avoid surprises.
As a parent and someone who watches ingredient trends, I notice the same pattern with many substances: fear shows up first, facts have to catch up. Propylene Glycol Diacetate doesn’t hide any obvious nastiness at levels used for personal care, but ongoing research and open communication matter more than ever. The safest ingredient will always be the one that suits your individual skin and your comfort zone, paired with brands that don’t keep secrets about what’s inside.
Propylene Glycol Diacetate, often shortened as PGDA, shows up in plenty of places, from paints and coatings to cleaning products. I still recall the first time I came across it in my line of work. It wasn't a fancy science lab, but a busy production floor where solvents and emulsifiers determined product quality. The workers talked about PGDA as a go-to solvent. It didn’t give off a strong odor like some others. Right away, I noticed you barely caught a whiff of anything sweet and fruity, thanks to its mild, almost unnoticeable scent.
PGDA looks and acts like many familiar glycols, except with a few tricks up its sleeve. It pours out as a colorless, clear liquid. The density hovers around 1.08 g/cm³, which means it’s a touch heavier than water. Pour some into a glass and you won’t see it mix right away, since it won’t readily dissolve in water. People often seem surprised to learn it's got only partial solubility, so it behaves somewhere in between—won’t float on top, won’t sink, but needs a push to blend.
Its boiling point lands around 190°C. Most household stoves can’t reach that, so it’s easily stable in daily use. A quick test at the bench—leave a jar out in a warm office—and it hardly evaporates. The evaporation rate matches that of propylene glycol acetate. Its viscosity also means it won’t splash around too much, which I noticed once during a rushed clean-up job; you can mop up spills quickly with absorbent material and a glove, since it won’t seep through cracks as fast as thinner solvents. So, in industrial settings, it's a safer bet for reducing unwanted vapors.
On the chemistry front, PGDA remains stable during regular storage and handling. It won’t break down until you bring in a strong acid or base or heat it far above 190°C. It can hydrolyze over time, forming acetic acid and propylene glycol—especially if exposed to moisture. You definitely notice acetic acid if hydrolysis happens, because it gives off a much sharper scent. Its flash point sits around 83°C, so it’s not as risky as volatile solvents like acetone, but I wouldn’t leave an open container near hot equipment.
Companies pick PGDA for its balance—it doesn’t react with most coating components, won’t rust steel or aluminum, and stands up to everyday bumps and knocks in manufacturing settings. In environmental terms, PGDA breaks down in the presence of sunlight and soil microbes. It won’t hang around for years in the environment, which matters in places where waste management gets complicated. The EPA lists it as having low aquatic and human toxicity at levels found in finished products, which reassures those of us trying to balance effective performance with environmental safety.
Everyday use of PGDA matches up with demands for safer, more predictable industrial chemicals. I’ve watched paint manufacturers shift from fast-evaporating, smelly solvents to PGDA because workers complain less of headaches or nausea, and it helps coatings flow and cure smoothly. I do see a few issues: incorrect disposal or storage can still lead to spills, particularly in smaller plants without proper training. Wide education campaigns about safe handling, personal protective equipment, and disposal procedures can make a huge difference.
As regulations keep tightening around chemical emissions, PGDA often stands out as a step in a healthier direction. Engineers and product designers who learn the fundamentals—solubility, stability, breakdown products—find themselves better equipped to spot problems early and keep both workers and the planet safer.
Anyone who’s spent a little time around chemical storage knows that taking shortcuts rarely ends well. Propylene Glycol Diacetate sits on plenty of safety data sheets, but those lists collect dust until somebody wants to cut corners. Strong experience, not just best-practice posters in a breakroom, saves people from headaches and worse. Keeping this solvent safe means attention, not just compliance.
Every time someone ignores a product’s flash point, it opens the door for trouble. Propylene Glycol Diacetate doesn’t burst into flame on its own, but it’s still a flammable liquid with a flash point around 73°C (163°F). Workers in hot warehouses or chemical plants need to respect that limit. Storing drums or containers in a shaded, well-ventilated area drops the risk of vapors reaching dangerous concentrations. Racked barrels and clear labeling help as well. Tucking containers away from direct heat sources—steam pipes, sunlight, process equipment—makes a difference.
Breathing in solvent vapors can cause anything from a headache to long-term damage. Some workers turn up their noses at the idea of fans and fume hoods—until a lack of fresh air puts them in a daze. Air circulation stops vapors from pooling at nose level, especially in indoor spaces or low-lying work areas. Installing explosion-proof exhaust fans isn’t optional in rooms where fumes could gather. The fresh air isn’t just about comfort—it keeps everyone sharp and accident-free.
Packaged the right way, even accident-prone warehouses stay safer. Tight-sealed drums with proper gaskets keep out contaminants and stop leaks. Leaking solvents damage more than floors; they poison soil and stress first responders. This isn’t theoretical—studies on chemical plant spills show that nearly half result from poor storage or old containers.
Absorbent pads, spill kits, and a clear cleanup process let workers respond without panic. Practice, not just paperwork, keeps reactions smooth. Any spill, large or small, deserves full attention and an immediate cleanup. Chemical compatibility charts matter: Propylene Glycol Diacetate shouldn’t share space with strong acids or oxidizers if you want to avoid unwanted reactions.
Even the best containers mean little without good labels. Readable hazard warnings, properly formatted across every drum and transfer vessel, matter far more than dreaded audits. The right gloves—usually nitrile, not latex—help protect skin exposed during transfers or drum-opening. Splash goggles shield the eyes, not just standard safety glasses.
Training goes beyond checking a box on a safety sheet. Anyone working with Propylene Glycol Diacetate should understand the health risks, emergency procedures, and the reasons behind every safety measure. Stories from the shop floor drive home the lesson much better than another slide in orientation. Sharing close calls creates real awareness.
In a world where supply chains stretch for thousands of miles, trusting a supplier’s practices matters nearly as much as getting the job done. Asking suppliers for recent inspection reports, understanding their transport logistics, and checking their reputation in the marketplace all signal a focus on reliability. Those who consider storage and safety a minor detail don’t stay in business long.
Propylene glycol diacetate shows up in all sorts of places—from paints and coatings to pesticides. Manufacturers like it for its solvent properties, and you’ll sometimes see it hiding behind the scenes in products that need a little chemical muscle. But in recent years, more folks have raised questions about its lasting impact on the land and water around us.
Plenty of chemical names out there get a bad reputation, so I took a closer look at what actually happens after propylene glycol diacetate leaves the factory or farm. Research paints a fairly clear picture: microorganisms in water and soil can break it down. The main reason for this is its chemical structure—this compound includes ester bonds, which bacteria and fungi tend to munch on pretty easily.
Many studies on biodegradability use tests developed by groups like the OECD. In these studies, propylene glycol diacetate meets the criteria for being “readily biodegradable.” Researchers observed that over 60% of the substance degraded within 28 days, which is the threshold for this kind of classification. I’ve looked at toxicity screenings too, and aquatic organisms handle it reasonably well in limited exposures, not showing chronic-level problems.
Personal experience with backyard compost, and even some industry recycling programs, show that not all chemical names spell disaster for our plots and rivers. Unlike some older solvents that linger for ages, propylene glycol diacetate doesn’t build up in plants or animals, so there’s less concern about bioaccumulation.
Despite many positives, not every story about propylene glycol diacetate is a rosy one. Commercial use often means larger quantities released in one go—from manufacturing spills, run-off, or improper disposal. Heavy loading can swamp soil and water systems, and bacteria hit with too much at once may slow their work. Some breakdown products, such as acetic acid, can cause pH changes in water, which stresses aquatic life in small ponds or streams.
Field samples from areas with frequent chemical releases show that local wildlife may face short-term disruption, even if the risks remain low over the long run. I have seen fish kills from other, less friendly formulas that saturated ponds beyond what bacteria could handle. We don’t have extensive real-world data on propylene glycol diacetate, but the worry is not misplaced.
Most evidence so far says propylene glycol diacetate offers a better option for industries trying to lower their pollution footprints. Many alternatives linger for months, poison fish, or slip up the food chain. Substances that break down this quickly help keep many pollution issues contained, especially when users follow guidelines for safe disposal.
But as someone who values both clean rivers and practical solutions, I see room for smarter safeguards. Coverage in factory yards, better leak controls, and clearer consumer labeling all bring risks down further. Using smaller, safer containers cuts accidental mishaps. Even industries committed to biodegradable options benefit from checking effluent for breakdown byproducts.
Green chemistry keeps moving, and that’s a good thing. Checks like Life Cycle Assessment could paint a more complete picture, covering raw material extraction, production, use, and final breakdown. Indepth monitoring around facilities keeps us honest—if rivers near chemical plants show little change, we’re probably on the right path. But a truly green product earns its tag by leaving soil, water, and creatures as undisturbed as possible, no matter how useful or “mostly safe” it is in a lab study.