Dipropylene Glycol Butyl Ether’s journey runs alongside the rise of modern chemistry and industrial cleaning. Once researchers moved beyond simple alcohols, the discovery of glycol ethers shaped paint, coatings, and cleaning industries. Companies needed stability, low volatility, and performance all in one. The discovery of these multi-ether compounds opened up better cleaning, safer workspaces, and reliable results. By the mid-twentieth century, production of glycols like DPGME and DPGBE scaled up across the globe, brought along by the demands of growing manufacturing and urban spaces filled with surfaces needing constant care. From my experience in specialty chemical distribution, it’s the less-glamorous ingredients like DPGBE that quietly power entire sectors.
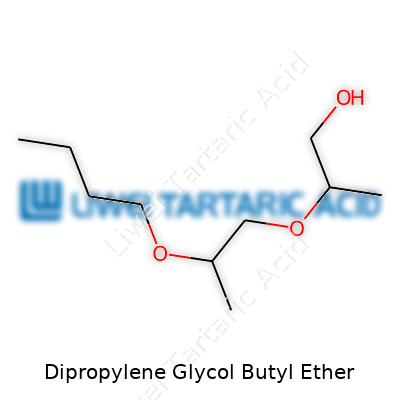
Dipropylene Glycol Butyl Ether hides behind the curtain, doing its job quietly in many cleaning formulas, paint thinners, and coatings. A glance at most safety data sheets won’t reveal marketing buzzwords, but look closer and you’ll notice this clear, odor-mild liquid repeatedly listed. The compound carries the chemical formula C10H22O4 and its structure features a mix of ether linkages and a butyl tail. Manufacturers often appreciate how it can dissolve both polar and non-polar soils, which widens its value far beyond “just another solvent.”
Pouring Dipropylene Glycol Butyl Ether into a beaker, you’ll notice its almost water-like viscosity, but a slightly oily feel lingers. This liquid stays stable at standard temperatures and resists evaporating quickly, reducing hazards from inhalation or loss of solvent. The boiling point sits around 230°C, meaning it can stand up to heat in industrial uses. Water solubility rests in a midrange sweet spot—neither too soluble nor too hydrophobic. Its moderate reactivity lets it blend into detergents, coatings, and inks without notably breaking down or causing unwanted side reactions.
Lab bottles and shipment drums display details: purity beyond 96%, water content below a percent, and color nearly colorless. For commercial use, safety data sheets highlight its compatibility with water and oils, flash point of approximately 100°C, and vapor pressure low enough that air concentrations rarely spike indoors. Labels. warn about skin and eye irritation, so gloves and goggles always makes sense, even for routine lab work. International shipping regulations classify DPGBE as non-dangerous, but sites that store bulk amounts follow extra fire safety.
Dipropylene Glycol Butyl Ether comes from reacting dipropylene glycol with butanol through an etherification process under acidic conditions. From feedstocks to reactor, the process relies on controlling water content, temperature, and agitation so the ether groups connect efficiently. Manufacturers keep waste streams minimal and routinely recover unreacted butanol for reuse. In my visits to chemical plants, optimizing this balance saves not just resources, but also cuts down on air emissions and costs.
DPGBE stands up well on its own, but chemists sometimes tweak it by modifying the butyl group or introducing additional ether links for specialized cleaners or industrial fluids. These modifications often fine-tune how easily the solvent breaks down oils or interacts with surfactants. In making blends for inks or coatings, formulators target evaporation rates and solvency curves, drawing from years of experimental data. My time working with development teams showed how even a small composition tweak can turn a mediocre product into a best-seller, whether it’s about faster drying paint or residue-free degreasers.
You might see “DPGBE,” “Butoxy Dipropylene Glycol,” or “Dowanol DPnB” in technical catalogs and product literature. Each company brands this glycol ether differently, but the chemical core stays the same. While multiple synonyms crop up—like “Propylene Glycol Butyl Ether, mixed isomers”—the industry recognizes the value in sticking to the basics and making sure anyone in the supply chain can identify the substance quickly.
Working with Dipropylene Glycol Butyl Ether, safety demands the basics: gloves, goggles, and good ventilation. It doesn’t set off alarm bells for flammability or chronic toxicity at practical exposure limits. Overexposure might mean red skin or stinging eyes, so keeping containers closed and handling spills quickly makes a big difference. Regular workplace monitoring protects operators against chronic low-level exposure. Companies check local and international regulations, but across Europe, the US, and Asia, similar standards highlight both chemical hygiene and responsible storage. In my own experience, keeping clear procedures in place and training staff pays off—companies face fewer incidents and less downtime.
Manufacturers trust DPGBE to lift grease and stains from floors, dissolve polymers in paints and inks, and help cleaners rinse away marks that refuse to budge. In auto shops, janitorial carts, and print houses, the solvent glides unnoticed through spray bottles and bulk tanks. Chemical plants prize its inertness and chemical stability in both surfactant and resin formulations. In my interactions with procurement teams, cost, consistency, and reliable supply matter as much as technical performance, so established logistics always stays high on the agenda.
Scientists continue to push the envelope with DPGBE. Today’s research explores safer, more efficient blends by pairing glycol ethers with bio-based surfactants or water alternatives. Formulators look for combinations that cut cleaning time or bring down costs without adding risk for workers. Environmental testing focuses on biodegradability, encouraging producers to tweak the glycol backbone or butyl chain for greener performance. In my own work supporting innovation teams, hearing about ways to drop VOCs or reduce residues always grabs attention since customers demand safer workplaces and less stress for end-users.
Researchers test DPGBE heavily for acute and chronic effects. Animal studies reveal low oral and dermal toxicity, with irritation the most common impact. Regulatory reviews across the globe, including the US EPA and EU REACH registries, echo these results. Chronic exposure risks remain low at practical industrial concentrations, but the industry doesn’t rest easy. Inhalation studies and ecological testing continue, and updates may prompt manufacturers to adjust formulas or recommend better PPE. People who’ve handled bulk glycol ethers understand respecting MSDS protocols and never getting careless even with “low hazard” tags.
As environmental rules tighten, DPGBE stands at a crossroads. Water-based formulations continue replacing straight solvents, and the pressure to cut hazardous air pollutants means chemists must rethink glycol ether usage. New research explores renewable sources and alternative methods to produce these versatile ethers, pushing for smaller carbon footprints and less waste. The next few years will likely see stricter sustainability demands paired with digital supply chain management, keeping DPGBE relevant where safety, solvency, and reliability can’t be compromised. Listening to customers in the coatings and cleaning sectors, it’s clear that the future will reward those who provide the right performance and smart compliance.
People grab all-purpose cleaner from store shelves and rarely wonder what makes it cut through stains. Dipropylene glycol butyl ether, which sounds pretty mysterious, shows up in quite a few cleaning and industrial products. This chemical helps dissolve grease and oil, making cleaning up messes a lot less backbreaking. Cleaners for glass, kitchen counters, car interiors, and even graffiti removal sprays often use it as a main workhorse. Without this compound, folks would spend a lot longer trying to wipe away soap scum or kitchen grease.
Paint goes on smooth because companies use solvents that control how fast it dries and how even it looks once finished. This chemical steps in here, too. If you’ve ever worked with latex paint and gotten a nice, streak-free coat in one go, it probably had help from dipropylene glycol butyl ether. The solvent keeps pigments and resins mixed together, letting them flow evenly when you roll them on a wall, door, or deck. People who paint houses for a living rely on this for a consistent result.
Factories that make inks for packaging, printing, or even for use in computer printers depend on solvents like dipropylene glycol butyl ether to keep colors bright and smooth. In the textile world, dye houses go through rivers of solvent in their bid to get color to grab onto every fiber. The chemical also shows up in metal cleaners before and after fabrication. Shops use it to prep surfaces, making sure welding and soldering work without hiccups.
Many chemicals that do the dirty work in industry also raise old questions about safety for workers and for the environment. People working around this solvent need gloves and eye protection, since skin exposure brings problems and breathing in the fumes over long stretches isn’t healthy. The U.S. Environmental Protection Agency keeps tabs on it, but findings so far show pretty low toxicity for folks who only meet it through household cleaning products. Big cleaning brands publish safety data sheets so users know where they stand.
The push for greener cleaning means companies experiment with replacements. Solvents from plants, like corn alcohol and citrus-based cleaners, keep popping up in stores. Problem is, natural options often cost more or don’t work quite as well, so most companies still use the proven stuff—sometimes mixed with milder alternatives to keep things safer and less smelly.
People worried about chemical exposure can check the labels and request safety information from manufacturers. Picking products with clear ingredient lists gives customers more control. Retailers and employers can choose options that carry third-party labels for low toxicity or environmental impact—eco-friendly badges aren’t just for show anymore.
Strong cleaning power usually comes with trade-offs. Makers and users both gain when they keep safety and performance in mind. Dipropylene glycol butyl ether isn’t going to disappear from store shelves overnight, because it gets the job done where others sometimes fall short. Still, as greener tech matures and more folks ask questions, the way industries clean and paint keeps evolving.
Many cleaning products, paints, and even some personal care items contain chemicals with complex names. Dipropylene Glycol Butyl Ether often shows up in the small print on bottles around the house or at work. People wonder what these chemicals do and whether they are safe to be around—or to put in contact with skin.
I remember sorting through spray bottles under the sink and squinting at labels, noticing there’s little plain-English help. Safety questions pop up fast. A little research shows that this solvent works as a cleaning agent, a degreaser, and helps dissolve stubborn grime. It doesn’t give off strong fumes and doesn’t have the harsh odor many associate with powerful cleaning agents.
Looking through safety data, Dipropylene Glycol Butyl Ether stands out for having lower toxicity compared to older solvents. Tests by the US Environmental Protection Agency and studies in Europe show this chemical breaks down in the environment and does not usually build up in the body. In routine exposure—like wiping a counter or washing a floor—most people aren’t exposed to levels that trigger health problems.
Companies and safety groups do caution about eye and skin contact, especially with undiluted or industrial-strength versions. Direct contact for a long time may cause irritation in some people. If swallowed in large amounts or inhaled in a workplace accident, it could make someone sick. Still, judged by how it’s used in households, these risks remain pretty small.
Some jobs involve more intense contact with solvents. Janitors or factory workers who use barrels of cleaning solutions need better protection and training. The average person using a bottle of bathroom cleaner once a week will usually only face short-term, low-level exposure. Gloves can help, especially for those with sensitive skin, but the main point is to avoid drenching hands or splashing in eyes.
Regulators have reviewed health data and don’t single out Dipropylene Glycol Butyl Ether as a major health threat, when used as intended. Accidents or misuse raise risk, not routine cleaning. For people with asthma or strong chemical allergies, even a gentle solvent could stir up a reaction, so choosing fragrance-free or “green” products—many of which still use this solvent—may help.
Interest keeps growing in cleaning and personal care options with fewer side effects. People want to know where their products come from and believe companies should be upfront. Detailed labels with clear language help consumers make choices without needing a chemistry degree. Manufacturers can replace older, harsher chemicals with ones like Dipropylene Glycol Butyl Ether—and aim for formulas that deliver results without putting people at unnecessary risk.
Taking common-sense steps matters. Use gloves if skin gets irritated, keep products out of reach of children, and make sure rooms are well-ventilated during heavy cleaning. People can support companies that invest in safety research and demand honest answers on chemical safety. With that, safer day-to-day cleaning habits become possible—without trading effectiveness for health.
Anyone working around paints, cleaners, or certain personal care products has likely crossed paths with Dipropylene Glycol Butyl Ether. In the lab, the liquid looks clear and colorless—a bit heavier than water. Pour a small amount, and there's hardly a whiff; the scent comes across as mild and nowhere near as sharp as old-school solvents like acetone. Unlike some chemicals that need careful locking away from sunlight or air, this solvent stays stable in typical storage conditions. It doesn’t flare up easily, with a flash point at about 81°C, so you won’t see it catching fire from a stray spark at room temperature.
What you notice first is how smoothly it blends into water and plenty of common organic liquids. Try diluting it, and you won't see any cloudiness or splitting—just a seamless mix. That solubility sits at the core of why cleaners and coatings manufacturers like it. Density runs between 0.95 and 0.96 grams per milliliter, so if you spill some next to water, you’ll find it sinks slower than syrup but doesn’t float like some oils.
Vapor isn’t something you deal with much. In a well-ventilated shop, it barely evaporates; this low volatility means workers aren’t breathing it in the way they might with older glycol ethers. Boiling kicks in at about 230°C; if you’re running it through a process line, you don’t need aggressive cooling systems to keep it in check.
I’ve seen Dipropylene Glycol Butyl Ether take on tough jobs. The chemical structure provides both a hydrophilic (water-attracting) and hydrophobic (oil-attracting) side. This balance lets it lift grease or break down stubborn residues without wrecking delicate surfaces. That’s important in industrial settings, especially when safety and protection of machinery surfaces rank high. Paint strippers and water-based coatings tap into this property. You get powerful cleaning or even spreading action, without the harshness you might expect from such effectiveness.
Reactivity doesn’t pose headaches for most handlers. Under normal shop conditions—no need for strong acids or excessive heat—it keeps its cool. Acid or base can break it down, but everyday operations rarely push it to that limit. If a spill lands in a drain, it’ll generally move through water systems with limited risk of creating dangerous byproducts, though good practice calls for responsible disposal. The U.S. Environmental Protection Agency points to low acute toxicity for humans. Eye or skin contact might trigger mild irritation, but the dangers prove much lower than heavy-duty solvents used in decades past. Studies suggest it breaks down in the environment at a reasonable rate, cutting down on long-term soil or water harm.
Factories, workshops, and even households continue to look for ways to reduce employee exposure without dialing back cleaning power. Dipropylene Glycol Butyl Ether helps achieve that balance. The industry moved away from stronger, more hazardous glycol ethers because workers reported headaches and breathing issues, especially in cramped or poorly ventilated spaces. Real-world research continues to back up the lower risk profile of this chemical.
Switching over to less volatile, more biodegradable solvents pays off by reducing air pollution and cutting down on waste meant for special treatment. Companies that train staff to use gloves and basic PPE often sidestep any irritation risk. Proper labeling and storage, along with spill kits on hand, round out a practical chemical safety approach.
Dipropylene Glycol Butyl Ether won’t fix every challenge in cleaning or coatings, but it reaches a solid middle ground for safety and performance. By paying attention to those physical and chemical traits, businesses and individuals both can weigh the benefits and make smart decisions for health and the environment.
Dipropylene glycol butyl ether shows up in a range of household and industrial products. From my years working around chemical storage, I know safety depends on focusing on both common sense and best industry practices. A chemical may look harmless at a glance, but ignoring safe handling can turn a routine job into a health hazard.
I’ve watched good products go bad just from sitting on a shelf too long, or sitting somewhere too hot. Like other glycol ethers, dipropylene glycol butyl ether breaks down under certain conditions. Heat, sunlight, or poor ventilation speed this up. Vapors sneak out, especially if you leave containers exposed or improperly sealed. Keeping it away from direct sunlight and storing it at room temperature reduces those risks.
Real trouble often shows up when storage areas crowd together chemicals that don’t get along. Oxidizers, strong acids, and bases react with glycol ethers. This means, in practice, labeling shelves matters as much as following the SDS. Using strong containers with tight lids, storing them upright, and keeping them away from incompatible materials becomes a matter of good housekeeping rather than just ticking off a regulatory box.
I’ve learned to respect the warnings on gloves and goggles. Skin and eyes can get irritated just by a careless splash, even if the liquid itself seems benign. It’s tempting to skip gloves when you’re in a rush, but personal protective equipment saves you pain later. In places with poor airflow, vapors build up without notice. So, using local exhaust or keeping a window open helps a lot.
Spills happen, especially during transfer. When I see someone try to mop one up with paper towels or rags, I think back to advice given to me early on: have absorbent material designed for chemical spills ready, and deal with the mess right away. It also pays off to keep a well-stocked spill kit close to the storage area.
Health issues linked to glycol ethers have shown up in workplace studies—skin issues, headaches, even more serious long-term effects if exposure happens over time. Consistent monitoring cuts down the risk. Training workers to pick up signs of leaks, make safe transfers, and report unsafe conditions ensures everyone goes home healthy.
Waterways take a hit too when chemicals get flushed down the drain. Waste disposal should always follow local regulations. Coordinating with licensed waste handlers, making sure chemical waste goes in the right container, and not dumping wash water pays dividends for both the environment and the company’s reputation.
Any business that keeps or uses dipropylene glycol butyl ether has a responsibility to stay up to date. Equipment wears out, storage rules change, and staff turnover means retraining happens often. Management should regularly check labels, replace worn PPE, and schedule training. Good practice starts at the top—with clear policies and follow-through.
From direct experience, the difference between a safe workspace and a dangerous one often lies in the small daily choices: storing products well, handling them with care, and treating every chemical with appropriate respect.
People often trust cleaning and industrial products without much thought about what happens to the chemicals after rinsing them down the drain. Dipropylene Glycol Butyl Ether, or DPNB, crops up in products for paints, cleaners, and coatings. Many companies tout its low odor and decent solvency power, but questions linger about the kind of environmental mark DPNB leaves behind.
Most cleaning chemicals eventually flow into waterways or seep through soil. If a chemical does not break apart easily through natural processes, then it has more chances to harm plants, animals, or even people. Based on current research, DPNB does have some claim to being biodegradable. In controlled studies, microorganisms can degrade it over time, which suggests it will not linger forever. Still, calling it “environmentally friendly” pushes things.
Laboratory results rarely capture all the flavors of the real world. Many countries have studied DPNB under strict test conditions and noticed it often breaks down under aerobic (oxygen-rich) conditions. But not every river, lake, or landfill sports the same tidy setup. The speed and completeness of biodegradation shift widely based on factors such as temperature, microbial life, and oxygen levels. Cold or polluted waterways can slow the process, letting the compound travel further and persist longer.
Data from the European Chemicals Agency shows DPNB tends to have a low bioaccumulation potential, which means fish and wildlife do not build it up in their bodies as much as with some older solvents. That said, low bioaccumulation is just one piece of the puzzle. You still have to look at how quickly it disappears and what by-products form along the way.
Some of the biggest concerns about so-called “green chemistry” involve companies leaning too hard on buzzwords. Many labels slap on the word “biodegradable” because it looks good on the shelf, but the details matter. Short laboratory studies may show DPNB breaking down by half in a couple of weeks under ideal conditions, but wastewater treatment plants or storm drains have to handle a swirling mix of other chemicals and stressors that change the game.
The U.S. Environmental Protection Agency puts DPNB in a class with low acute toxicity, so a spill will not turn a river toxic overnight. Still, repeated low-level exposures add up. Some aquatic species show minor stress signs at higher exposures, and mixing DPNB with other household chemicals can increase risks no lab test predicts. As someone who has scrubbed many floors and spent time in industrial settings, it becomes obvious that labels do not always match the reality of how we use products or how they flow into the environment afterward.
Chemists and manufacturers constantly look for ways to balance performance, safety, and price. For someone looking to reduce chemical load, small changes help more than hunting for miracle substitutes. Using less, switching to plain soap or vinegar where possible, and backing brands that share full ingredient data all help limit the mess we leave for the next generation. Community support for stricter labeling and transparent testing keeps pressure on companies to improve.
DPNB walks the tightrope between convenience and long-term health of the planet. Most data point to it being less harmful and more likely to degrade than the harshest solvents of past decades, but calling it harmless would be a stretch. Awareness and careful habits always beat fancy claims stamped on a bottle.