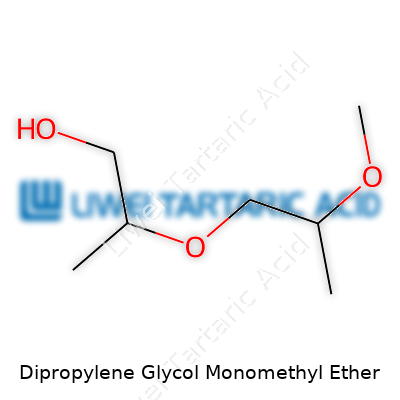
Dipropylene Glycol Monomethyl Ether emerged in the twentieth century alongside the push for safer, more adaptable industrial solvents. Back then, people relied heavily on simple alcohols, esters, or glycols for cleaning, paint production, and inks. The rise of environmental regulation made folks take a longer look at health impacts and flammability risks. Researchers responded by tweaking common glycolethers, searching for alternatives that balanced low toxicity, manageable volatility, and effective solvency. This drive for cleaner, worker-friendly chemicals led chemists to play with propylene oxide and methyl alcohol under controlled conditions, ending up with numerous isomers grouped together as Dipropylene Glycol Monomethyl Ether—sometimes called DPM. By the 1970s, manufacturers started trading it under familiar brand names, offering industry a solvent that avoided the headaches and watery eyes often triggered by stronger options.
Ask anyone in coatings, cleaning, or electronics, and Dipropylene Glycol Monomethyl Ether turns up as a real workhorse. It usually appears as a clear, nearly odorless liquid sold in bulk drums or intermediate containers. Producers highlight its medium evaporation rate, which helps film formers settle without trapping too much dust or slowing down productivity. Companies like Dow, BASF, and LyondellBasell stamp their trademark names on it—DOWANOL DPM, Arcosolv DPM, or Glycol Ether DPM. Regardless of the logo, the product blends swiftly with water or standard solvents, showing solid stability against acids and bases. Most buyers in the market look for consistent purity and traceability from plant to client site, reflecting the attention regulators and buyers give to health, safety, and transparency.
Dipropylene Glycol Monomethyl Ether acts like a bridge between water and oil, pulling grease off metals while washing away with a simple rinse. A typical sample carries a boiling point above 180°C, a flash point that keeps most workplaces out of the danger zone, and a viscosity just thick enough to avoid surprise spills. Vapor pressure sits low enough to minimize indoor air build-up even in poorly ventilated corners. It dissolves resins, dyes, and polymer chains with surprising ease, which explains why finishers and paint chemists turn to it instead of stubborn, slow-drying glycols. Left open to the air, it barely raises a whiff compared to other cleaning agents, marking a welcome change for workers tired of headaches or itchy nostrils.
Every container of Dipropylene Glycol Monomethyl Ether carries clear labeling about water content, purity, and presence of isomers. Most specifications demand the main component reach at least 99% concentration, limiting water below 0.1% and acidity in the ppm range. Labels feature hazard pictograms according to GHS rules—paying attention to irritant warnings even if outright toxicity remains low. Shipping documents spell out UN codes and recommendations for respiratory or splash protection. Production facilities line up with ISO and REACH protocols, and buyers clutch certificates of analysis for every delivery. Formulators and EH&S coordinators lean on these details each time they approve a new batch for use on the line.
Manufacturers make Dipropylene Glycol Monomethyl Ether by reacting propylene oxide with methanol in a basic or acidic medium. That reaction tends to offer a blend of isomers since propylene oxide can attach to methanol in different spots. Skilled chemical engineers keep a close eye on reaction temperatures and ratios. They feed the resulting mixture into a distillation column, which lets them pull off the isomer blend at just the right point—long before heavy byproducts or leftover starting material spoil the outcome. Any top-tier plant recycles solvent residues and cools vapors to limit waste, often piping unused streams back through the process.
Once off the line, Dipropylene Glycol Monomethyl Ether resists most heat or oxidation attacks, so it rarely breaks down unless pushed by a strong acid or base. Under lab conditions, chemists can swap its methyl group for other short alcohols, creating specialty glycolethers for niche coatings or inks. It stays inert in the presence of most resins—which matters when formulating paints or adhesives that need a stable carrier. Sometimes research teams modify this ether by attaching extra glycol chains, tuning evaporation or water affinity for ultra-fast drying or residue-free wipes. Anybody experimenting here balances cost and performance, not to mention the ever-present scrutiny of safety and regulatory teams.
In practice, Dipropylene Glycol Monomethyl Ether goes by several names. Besides CAS 34590-94-8, brands use designations like DPM, Glycol Ether DPM, and Propylene glycol monomethyl ether (mixture of isomers). Importers and distributors rely on these titles for inventory tracking, MSDS referencing, and regulatory filings. Trade associations standardize these identifiers, making it easier for customs agents and emergency responders to recognize what’s inside every barrel or tote.
Safety teams stress gloves and splash goggles as standard gear for working with Dipropylene Glycol Monomethyl Ether. While it doesn’t burn skin or cause acute health crises at low exposure, some users experience mild eye or respiratory irritation—especially in tight spaces with poor air flow. OSHA and ACGIH set exposure guidelines, flagging routine ventilation and spill management as must-haves for busy plants or cleaning crews. Anyone in transportation checks containers for leaks before stacking, and spill kits hang within arms’ reach for the occasional knock or tear. Fire marshals rate DPM as having a moderate fire risk, so warehouse managers enforce no-smoking rules and keep ignition sources far from storage racks. On the compliance front, REACH and TSCA filings keep regulators in the loop on hazards, emissions, and safe use guidelines.
Industries flock to Dipropylene Glycol Monomethyl Ether for more than just cleaning metals. Paint formulators build it into latex, acrylic, and alkyd systems to slow drying for a glass-smooth finish that holds up under sun and rain. Printing presses use it to prevent paper jams and mottled graphics by leveling ink layers. In electronics, technicians rely on it for dissolving stubborn flux and prepping circuit boards without leaving greasy films or attracting dust. Custodial crews reach for it to clean floors and tiles—not least because it’s gentler on sensitive surfaces than old-school solvents like xylene or acetone. Even the fragrance world sneaks it into scent carriers, trusting its neutrality and low odor to preserve perfumes’ intended notes.
Every year, research labs spend time investigating lower-toxicity substitutes and greener production routes. One line of inquiry targets waste reduction, substituting bio-derived alcohols in place of fossil-fuel-based methanol or propylene oxide. Others build structure-activity maps, looking for tweaks that keep cleaning power up but lower VOC footprints in finished products. Toxicologists run animal and cell studies, measuring subtle impacts on hormone systems or chronic health. Industrial partners bet on process efficiency—finding catalysts and distillation tweaks that trim energy costs and waste. Technical conferences fill with debate about drop-in replacements or blends fine-tuned for surface tension and boiling range. The field pushes forward thanks to partnerships between big chemical players, university labs, and government agencies all pulling for safer, high-performing options.
Toxicity studies find Dipropylene Glycol Monomethyl Ether scored lower in acute hazards than many older solvents. Breathing high concentrations produces mild nose or throat irritation, but typical workplace exposure rarely pushes levels high enough to cause harm. Lab rats exposed over longer stretches showed no clear cancer link or birth defects at workplace concentrations. Skin contact tended to cause redness for only a small subset of test subjects. Environmental impacts stay small since the compound breaks down in wastewater and doesn’t accumulate in fish or plants. Even so, industry keeps a close watch and regulatory scientists chase after subtle effects—aware that small risks can grow if production volumes jump or user habits shift. Responsible handlers stick to proven safety practices, especially in jobs where hundreds of gallons move each day.
As industry trends trend toward green chemistry and worker wellness, the future of Dipropylene Glycol Monomethyl Ether depends on following tougher safety and emission standards. Process engineers continue to adapt, aiming for cleaner syntheses that use renewable feedstocks and generate less pollution. Lifecycles get tracked from cradle to grave, pushing every supplier to dial down risks from factory gate to disposal or recovery. Consumer groups watch for any new evidence of chronic health effects, and regulators push companies to upgrade training and emergency planning. In fast-changing fields like lithium battery manufacturing and printed electronics, product developers experiment with DPM’s structure, looking for blends that keep performance high while offering easier recycling or safer air quality. The road ahead lies in smart chemistry, transparency, and a commitment to safer, more sustainable manufacturing across the board.
Walking through the supermarket aisles, there’s a good chance the cleaning products catching your eye make use of more than just soap and water. Dipropylene Glycol Monomethyl Ether, or DPM for short, finds a home in a range of solutions because it helps dissolve, spread, and mix other substances without much fuss. Most households grab a spray bottle for kitchens and windows—the clear finish, the way it cuts through grease, and the lack of streaks owes a lot to solvents like DPM. Not just some obscure chemical, but a practical standby for getting formulas working in a variety of ways.
Stepping deeper into what happens behind the scenes, painters and contractors use latex and water-based paints that benefit from this glycol ether. DPM lets colors glide smoothly and dry at a manageable pace. It’s less about fancy chemistry, more about getting a job done without strong odors or the risk of headaches. Many appreciate paint jobs that don’t leave the house smelling for a week, and DPM helps on that front. Cleaning after a messy job goes the same way—solvents built around DPM lift grime from metal parts or dissolve stubborn adhesives, making a difference for auto shops, factories, or anyone tackling a tricky cleaning job.
An ordinary bottle of lotion or perfume on the bathroom shelf often contains a solvent, and DPM fits in quietly. Lotions need to feel silky, with every ingredient staying mixed for months until you run out. The solvent keeps everything blended and stops perfumes from separating or degrading before they even reach your skin. It’s little details, like not having to shake a bottle every time before squirting it onto your hand, that come from careful ingredient choices. DPM delivers here without introducing much smell or irritation, which helps people who rely on gentle skin care formulas.
With widespread use comes the responsibility to look into safety. Reports from the European Chemicals Agency and the US Environmental Protection Agency give DPM a decent track record at normal handling levels. Industrial workers using DPM directly need gloves and good ventilation, just as with most solvents, but ordinary folk who use finished products run into DPM at such small amounts that risk stays low. Some worry about any chemical in a bottle, often for good reason, but with DPM the focus usually lands on balancing effectiveness and gentleness.
Consumer demand keeps shifting toward greener cleaning and simpler skincare. Some companies look for alternatives derived from plants or start cutting overall solvent content. The challenge involves finding replacements that dissolve, clean, and blend as well as DPM, without losing the performance people count on each day. Regulations take a cautious approach, so any new substitute goes through safety checks just like DPM did years ago. The best progress comes from sharing research openly—university labs, regulators, and brands all testing side by side before making a switch.
In my own work with consumer product testing, DPM stands out because it bridges industry know-how and common sense needs at home—an ingredient that solves problems quietly, without showy claims. It’s that kind of reliability that shapes the things people reach for every day, whether they realize it or not.
Dipropylene Glycol Monomethyl Ether, better known as DPGME, shows up in a lot of places. It works hard in cleaning sprays, paints, and even in inks and personal care products. Every day, ordinary people splash windows with it, mix it into paint, or rely on it to break up grease and grime. I’ve seen labels with scary-sounding chemical names, and DPGME can certainly look intimidating if the only thing you know about it is the science jargon.
Most folks start to worry about a chemical once they spot one of those hazard warnings on a bottle. The National Institutes of Health and the Environmental Protection Agency have both studied DPGME. At household concentrations, it doesn’t seem to cause trouble when you breathe it in or touch it for short periods. OSHA sets safe exposure limits for workers, and DPGME doesn’t poison people who use it properly at home.
Scientists haven’t linked DPGME to cancer or birth defects at normal exposure levels. It doesn’t collect in the body over time, and in the air, it doesn’t stay long enough to build up indoors like some solvents. Skin irritation sometimes happens, mostly if someone gets a bunch on bare skin or keeps it there too long without rinsing. Eyes can sting if splashed directly, just like with most cleaning chemicals.
Most problems with chemicals like this don’t come from the chemical itself, but from how people use it. Proper ventilation helps—open a window or flip on a fan. I’ve always kept gloves nearby, especially with the stronger cleaners. Washing up after working with these types of products matters more than people realize. The product label gives clear advice, and it’s worth a quick read before diving in.
Mixing DPGME with other strong chemicals causes the biggest trouble. Ammonia or strong acids don’t belong in the same bucket. Following the directions and using common sense—like never drinking from a cup used for a cleaning project—keeps risks low. For folks with sensitive skin or asthma, even low levels might cause bother, so a little caution goes a long way.
DPGME isn’t banned in the United States or Europe. The Food and Drug Administration and European Chemicals Agency both agree it’s safe for its approved uses. You can check the EPA’s Safer Choice list for guidance on cleaners, or dig into the databases run by the NIH for more scientific detail. Reputable brands include safety data and respond to questions, which helps people spot quality products and weed out sketchy ones.
Some newer cleaners cut back on harsh chemicals with plant-based ingredients, but performance can slip for heavy-duty jobs. I like to see manufacturers providing full ingredient lists and providing less-hazardous choices. At home, storing chemicals securely, buying only what’s needed, and using proper gear make the difference. For workplaces, regular training and fresh air can keep everyone safe. Anyone with a reaction to a product should stop use and reach out to a doctor, not just shrug it off.
Dipropylene glycol monomethyl ether, often called DPM, finds its way into paints, cleaners, and industrial coatings. It handles a lot of mixing and blending but stays mostly behind the scenes. Many folks handling this solvent start off thinking it’s harmless because it doesn’t attack the senses like harsher chemicals. The reality is, it has a low odor and low evaporation, but treating it casually opens the door for safety slip-ups.
DPM does best in cool, well-ventilated storage rooms. Heat makes it more likely to degrade or just spoil, so dry places out of direct sunlight keep it stable. Any room with poor airflow risks vapor buildup, especially during a leak. Some practical experience: I once saw a workshop leave DPM drums in a sunlit corner for a week. Even after that short exposure, the drum bulged out of shape, which could have caused a real mess if the seal split. Store this solvent in steel or HDPE drums with tight lids, never in open containers. Keep them clear of acids and oxidizing agents — mixing these can spark chemical reactions, which nobody wants in the workplace.
Repeated contact dries out and irritates the skin, even without splashes. Gloves rated for organic solvents, goggles, and long-sleeved work clothing stop most accidents before they start. The times I got careless and used just regular latex gloves, the difference was clear after a day of refilling quarts or cleaning up spills — standard gloves break down too fast, offering almost no protection.
Fumes hang around longer in dead air. General room fans won’t move much vapor, so local exhaust or even a fume hood makes a big difference, especially in small shops or lab benches. DPM vapors can knock back concentration with enough exposure; headaches and dizziness aren’t rare. Use chemical-resistant mats for flooring, too. Contaminated shoes track solvent through workplaces, which is something everyone overlooks until clean-up gets out of hand.
Even small DPM spills coat surfaces, making floors slippery and dangerous. In shops I worked at, one ounce on concrete caused two slip-and-fall accidents in a single afternoon. Keep absorbent pads and spill kits close by — the minute DPM hits the floor, block foot traffic and start cleanup. Double-bag used rags or pads so vapor doesn’t linger in trash rooms. For skin contact, immediate washing with soap and water helps. No shortcuts.
Fire safety deserves respect. DPM won’t ignite as fast as naphtha, but it does burn — its flash point sits at about 75°C (167°F). Fire extinguishers rated for chemical solvents should always be within reach. Sprinklered areas provide another level of protection, but don’t get complacent just because the solvent isn’t classified as highly flammable.
Training goes further than any MSDS sheet. Everyone, not just managers, should know what a drum label actually means, how to spot leaks, and how to use spill kits. Ventilation and PPE aren’t optional extras for DPM, despite its mild odor. Storage rules only work when practiced. Regular audits and a hands-on approach to safety can cut down on emergencies.
DPM might seem tame at first glance, but it deserves the same respect shown to stronger chemicals. Secure storage, practical personal protection, and fast spill response keep workers safe and processes running smooth.
Dipropylene glycol monomethyl ether, known in shorter terms as DPM, often pops up in a regular workday for painters, cleaners, and people working in labs. Its biggest appeal is its ability to blend in with a long list of substances without much fuss. Pour some DPM into water and watch them merge with no obvious resistance. This isn’t just a neat science trick—it's the reason DPM helps deliver reliable results in industries that need consistent cleaning or gentle solvents for delicate surfaces. At home, DPM frequently tags along in household cleaners and paints, smoothing out the job of removing stubborn stains or evening out color on a wall.
The real magic starts at the molecular level. DPM features a chemical backbone that plays well with both water and a solid lineup of organic solvents. Think of it as a bridge between two neighborhoods—one with molecules that love water, another with those that avoid it. Because of this, DPM slides easily into water, alcohols, esters, and even some hydrocarbons. This means a cleaning chemist can drop DPM into their mix and trust it to keep other ingredients from separating. A painter can count on fewer streaks or uneven patches.
Many people get hung up on the complicated chemical jargon, but for those of us who just want paints that glide on evenly or cleaners that don’t leave chalky leftovers, the takeaway is simple: DPM carries its weight. Products relying on this solvent tend to perform well. Its compatibility makes life easier for manufacturers—they don’t have to engineer around finicky quirks. For environment-minded folks, this means formulas need fewer harsh additives, since DPM’s flexibility already brings stability.
Like any chemical, DPM isn’t a one-size-fits-all answer. There are limits. For folks working in enclosed spaces, heavy exposure can irritate the eyes or skin. Safe handling instructions stay front and center on product labels for good reason. In personal experience, sticking to gloves and decent ventilation makes a difference, especially after a long afternoon scrubbing down equipment or prepping a wall for repainting.
Wastewater treatment plants see a fair share of DPM in their loads. Biodegradation rates give environmental engineers some peace of mind—DPM doesn’t tend to hang around in nature as long as harsher solvents do. That said, there’s no excuse to pour leftovers down a drain. Smart disposal still matters. The chemical’s ability to mix widely means it can slip into local waterways unless caught during treatment, so following regional disposal rules makes everyone’s life simpler.
Training goes a long way. I’ve seen colleagues new to industrial cleaning underestimate the fumes from repeated DPM use. Supervisors who give real-world demos—ventilating rooms, treating small accidental splashes right away—set up safer workplaces. Updates to safety protocols keep up with shifting research, especially for companies experimenting with new blends. For home use, smaller packages and easy-to-follow directions cut down on misuse or accidental spills.
Formulations that rely on DPM can lean on its wide compatibility without maxing out concentrations. Diluting with water or companion solvents both stretches product life and reduces the punch per dose. This comes with a bonus: fewer raw materials used, lower emissions during both production and eventual breakdown.
DPM’s flexibility matters for the people using it and for the planet catching its leftovers. Blending it with water or other solvents opens up a range of safer, more effective products that don't sacrifice performance. Smart handling, transparent labeling, and responsible disposal keep those benefits in balance.
Walk into a hardware store, pick up a can of water-based paint, and there's a good chance you'll find Dipropylene Glycol Monomethyl Ether on the ingredient list. The stuff helps paints glide on walls evenly, preventing streaks and patchiness. It also slows down how quickly paint dries, giving people more time to work—especially important if you're not a professional painter and need those precious extra minutes to fix mistakes. On job sites, the way DPgME limits the amount of harsh fumes in the air matters. Painters, construction crews, and even homeowners benefit from lower odor and reduced exposure to solvents that can mess with your health in the long run. The Environmental Protection Agency pays close attention to what goes in paints, and this particular glycol ether often gets a pass for indoor jobs because it's less volatile than old-school solvents like toluene and xylene.
Anyone who has scrubbed a greasy restaurant stove or wiped down a smeared whiteboard probably used a cleaner that contains Dipropylene Glycol Monomethyl Ether. It's prized in janitorial circles and busy kitchens alike because it can dissolve greasy, sticky messes that plain soap and water leave behind. I spent some time in a commercial kitchen—when the shift wound down, the grime didn’t stand a chance. The cleaning crew could wipe up sauces, oils, and chocolate without leaving smears or residue. DPgME doesn’t just boost cleaning muscle, it also lets manufacturers keep the formulas low-odor and less irritating to skin and lungs.
Open a new magazine and the smell hits you right away. A lot of that aroma comes from the solvents in inks. Magazines, brochures, and packaging benefit from fast-drying, sharp-looking inks, and that's where Dipropylene Glycol Monomethyl Ether comes in. Printers like to use it because it helps inks dry at the right speed—enough to prevent smudging, not so much that the ink gets trapped in the rollers or blocks up the print heads. That keeps production lines running, prevents waste, and gets magazines onto shelves faster. Lower emissions from printing plants have become a big deal, and DPgME helps keep those numbers down, offering an edge over harsher solvents.
Check the ingredients on some hand sanitizers, lotions, or perfumes and you might spot Dipropylene Glycol Monomethyl Ether. It's part of what helps fragrances dissolve smoothly, so your lotion doesn’t separate or your perfume doesn’t clog the sprayer. In factories, consistency matters for big batches—customers want to know their moisturizer feels the same each time. My dermatologist once pointed me toward a hand cream with DPgME for my eczema. Turns out, a bit of the right solvent can blend active ingredients so they actually do their job. Plus, regulatory agencies tend to keep an eagle eye out for unsafe chemicals, so the continued use of DPgME in personal care backs up its safety record.
Not every solvent or ingredient gets to play a role across so many industries. For Dipropylene Glycol Monomethyl Ether, continued improvement in workplace safety and air quality standards offers both a challenge and an opportunity. As the push toward cleaner labels and greener chemistry ramps up, research keeps chasing new glycol ethers with even less impact on health and the environment. For now, DPgME remains a favorite thanks to its versatility, safety profile, and steady track record across multiple trades.