Some chemicals carve out a long, winding path. Dipropylene glycol monomethyl ether acetate didn’t just appear in laboratories overnight. Its origin runs back to the twentieth-century push for safer and more efficient solvents, right when chemists began craving alternatives to volatile, highly flammable options. The push came from stricter workplace standards and regulations, as well as the search for raw materials that would not cause massive fires or put workers in immediate danger. Over the decades, this solvent found its place in coatings, inks, and cleaning agents, surprising many who stick to older formulas. Watching industries adjust, it’s clear that regulatory pressure often drives invention just as much as curiosity or efficiency ever have.
Dipropylene glycol monomethyl ether acetate looks simple on lab paper but plays a big role across several industries. This clear, nearly odorless liquid sits quietly in warehouses and labs, ready for paint, cleaning, or electronics work. Companies find value because it bridges the gap between being gentle enough for delicate tasks but strong enough to dissolve what others cannot. Its influence stretches far, thanks to a few key traits—low volatility, high solvency, and respectable stability. Talking with folks in product development, those who know this solvent appreciate versatility, especially when working with changing formulations or strict client needs.
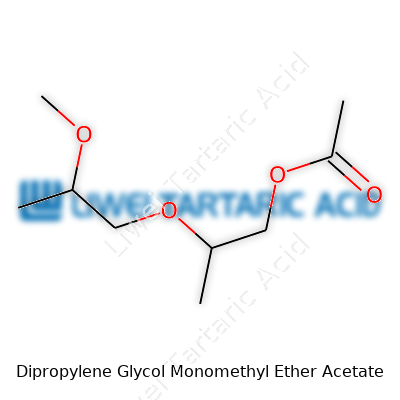
The physical look of dipropylene glycol monomethyl ether acetate rarely surprises anybody—colorless with a mild odor. Its boiling point hovers around 190°C, letting it stick around longer in paints and coatings without evaporating at the first sign of heat. Specific gravity falls between 0.96 and 0.98, heavier than water, which matters for blending and mixing. It dissolves in water and many organics, which opens doors for chemists trying to solve tough formulation puzzles, especially when developing coatings that need slow drying times or stable emulsions. The molecular structure keeps it stable; the balance of polarity and molecular weight guarantees it won’t run off or react without reason.
Regulatory agencies set strict bars for solvents, and manufacturers need to keep paperwork clean. The technical specifications cover purity levels, typically above 98%. Moisture content checks are standard, since even a bit of water can ruin entire batches in sensitive manufacturing processes. Hazard information labels cover flammability warnings, chemical identification, and emergency measures. GHS pictograms appear on every container. Product certificates don’t just keep companies legal—they drive trust. Customers tend to ask for full traceability, batch testing docs, and compliance certifications, especially after recent scares around counterfeit products slipping into supply chains.
Manufacturers produce this solvent in a few controlled steps. It starts from dipropylene glycol, which reacts with methyl acetate or similar agents. The reaction calls for a catalyst, usually acidic or sometimes basic, matched to the desired yield and purity. Reaction vessels need to hold consistent temperature and pressure, since swings throw off product quality or leave unreacted leftovers. After the esterification step, distillation follows to strip byproducts and unwanted water. Good operators watch overhead temperatures and vacuum levels carefully—all to preserve the acetate structure. Modern facilities invest heavily in monitoring systems to keep contaminants out, which makes a difference for downstream performance and workplace safety.
In labs, chemists often look for solvents that don’t react more than needed. Dipropylene glycol monomethyl ether acetate stays stable under normal use, but it can break down under strong acids or bases. In high pH or heat, you may see hydrolysis, which produces the parent glycol and methyl acetate. Most real-world uses avoid those conditions. When manufacturers want to tweak properties—say, for faster or slower evaporation—they may blend it with related compounds or use it as a carrier for tailored resins and polymers. Research teams like its steady nature, since it doesn’t introduce unexpected variables when mixed with functional additives for paints or adhesives.
Market shelves rarely stick with one name. Dipropylene glycol monomethyl ether acetate turns up as DPM Acetate, Propylene glycol methyl ether acetate, or even by trade names, depending on the manufacturer’s regional presence. In research papers, you’ll sometimes spot abbreviations like DPMA. This web of synonyms can confuse buyers, but savvy suppliers provide cross-reference sheets listing CAS numbers and international codes. Navigating regulatory paperwork, it becomes clear that knowing these aliases prevents shipment mix-ups or regulatory fines for improper documentation. Global trade demands a firm grip on these naming nuances, especially as companies ship barrel lots across borders.
Industry experience with solvents teaches one lesson clearly: training prevents disasters. While dipropylene glycol monomethyl ether acetate doesn’t rank as the most hazardous solvent, exposures above recommended limits cause irritation to eyes and skin. Ventilation matters in production plants and workshops, not only to keep workers comfortable but to reduce fire risk. Safety Data Sheets direct users to wear gloves, splash-resistant goggles, and sometimes full respirators in high-volume settings. Companies invest in spill kits and emergency response drills, since even low-toxicity chemicals can become dangerous at scale. Recent workplace studies found that strong policies lead to fewer incidents—a point not lost on safety managers hoping to build a culture where people speak up before hazards become accidents.
If you’ve ever watched paint dry on a car or door, chances are solvents like this one played a part. The coatings industry remains a major customer. Because dipropylene glycol monomethyl ether acetate lets paint film settle slowly, workers get a smoother finish, with less risk of blotching or pinholes. Electronics manufacturers lean on it for cleaning delicate circuit boards—its gentle solvency clears flux residues without eating through plastic or rubber. Printing ink suppliers appreciate its steady evaporation, which means sharper colors and steadier print runs. Professionals in cleaning, adhesives, and agrochemicals cite reliability and a broad solvency profile. Knowing how one chemical supports so many processes, it’s clear that the right solvent can speed up work, cut costs, and raise end-product quality in ways invisible to most end-users.
Innovation teams rarely rest. Companies keep searching for solvents with even lower toxicity and ecological impact. In recent years, R&D has focused on refining the synthesis process, aiming for higher yield, lower waste, and better lifecycle emissions data. Research into biodegradable alternatives picks up pace, driven by strict European and North American chemical regulations. Formulators sometimes blend this solvent with emerging green options, trying to balance environmental goals with customer performance demands. Insights from recent university studies help, revealing new reaction pathways, catalyst optimizations, and cleaner separation methods. Veteran chemists I’ve talked to mention that collaboration—not just competition—drives technical progress, especially when sustainability and safety top public agendas.
Long-term exposure studies provide peace of mind—or warning—depending on results. Dipropylene glycol monomethyl ether acetate fares well compared to older, harsher solvents, but regulators keep a close eye on chronic effects. Acute toxicity ranks low for skin and inhalation, though persistent contact leads to irritation. Animal studies show limited organ toxicity at expected workplace levels, but ongoing monitoring remains standard policy. Companies fund independent reviews, looking for early signs of bioaccumulation or long-term DNA damage. Growing interest in endocrine disruption and environmental persistence compounds the need for thorough risk assessments. Safety professionals rely on up-to-date findings to set workplace limits and update training.
The chemical industry faces mounting pressure from regulators and public opinion. Dipropylene glycol monomethyl ether acetate, with its established safety record, stands in a strong position—at least for now. Demand rises in fast-growing markets like Asia-Pacific, especially as infrastructure and automotive projects ramp up. There’s a push to tighten emission controls in factories using volatile solvents, which may boost interest in even slower-evaporating, lower-impact alternatives. New research could lead to modifications that enhance biodegradability or reduce carbon footprints even further. Industry insiders talk about hybrid solvents—mixes designed for specific tasks but with safety and sustainability built into their DNA. After decades of slow evolution, solvents like this one might soon see their biggest leaps yet, born out of necessity and smart science.
People don’t walk down the street and talk about Dipropylene Glycol Monomethyl Ether Acetate, but the stuff helps build the world around us. I first heard its name in a paint shop while hunting for a wood finish. The shop owner explained it’s not just the color or the can—it’s whether that paint even goes on the wall without drama. Under that tongue-twister of a name, there’s a liquid that keeps paint smooth, dries it slow enough to avoid streaks, and lets both professionals and DIY types put up clean coats. Every can of water-based paint relies on little chemical sidekicks like this one.
Across the construction industry, this solvent steps up as a helper inside paints, varnishes, and certain inks. Its main trick lies in dissolving pigments and keeping them from clumping, which is a bigger problem than most people think. If you’ve watched cheap paint chunk up or dry too fast, you’ve seen what happens when companies cut corners. Honest product labels list this chemical because it works—paint glides on evenly, stays wet long enough to even out brush marks, and doesn’t smack you with harsh fumes. Some artists and contractors know the difference after a single coat.
Chemists go after this compound for its balance. It’s strong enough to clean residues left behind by oils and waxes but doesn’t tear up skin or surfaces like heavy-duty solvents such as acetone. That means people using paint strippers or graffiti removers should check the ingredients; substitutes often end up being either ineffective or harsh. Environmental rules are getting tighter in many countries, which pressures manufacturers to seek out lower-odor and safer alternatives. This solvent sits in the sweet spot, offering the strength needed while also meeting many health requirements.
People inside the tech world depend on clean surfaces, especially in places where fingerprints spell disaster for performance. Working years ago in a print shop with circuit boards, I watched operators wipe them down with specialized cleaners, and this molecule kept reappearing on the safety labels. It flashes off at a predictable rate, which means it doesn’t drip or leave patches of moisture. That reliability attracts both engineers who assemble delicate electronics and machinists finishing plastics.
Using chemicals always opens a conversation about trade-offs. While this solvent offers lower toxicity than old-school choices like toluene, getting it on your skin or breathing too much isn’t wise. Workers in manufacturing need gloves and good ventilation. The broader conversation turns toward green chemistry—finding replacements that work as well but take less from the environment. Some have tried to swap in bio-based chemicals, though they often fall short in terms of cost or performance.
As regulations about emissions tighten, this compound could see tweaks to production, or companies may invent replacements that work just as well without building up in landfills or waterways. Until then, it sticks around for a simple reason: It helps get the job done without as many headaches. From home projects to the world’s sleekest electronics factories, it’s the supporting act most people never know about.
Dipropylene glycol monomethyl ether acetate, or DPM acetate for short, pops up in paints, coatings, inks, and cleaning products. Industrial settings see it quite a bit. It sounds technical, but we’re all likely to come across it at some point if a home is getting painted or someone works in manufacturing.
Workplaces using DPM acetate need clear information on risk. Breathing in fumes over a long workday might lead to headaches, dizziness, or a scratchy throat. People using this solvent in confined spaces might notice more coughing and even some nausea if the ventilation isn’t decent. In high enough concentrations, some folks report feeling drowsy or lightheaded. Eye and skin contact can also spark irritation, especially for people with sensitivities or allergic tendencies.
The data from research is straight: OSHA and NIOSH track the chemical and ask employers to keep air concentrations below strict limits. Animal tests show that, at large doses, this solvent messes with the liver and kidneys, though these cases reach doses much higher than most people see at work or at home. Long-term studies on human cancer risk haven’t shown clear links—so as it stands, experts do not call it a carcinogen.
My own hands-on experience has taught me that painting with products listing DPM acetate demands respect for the label. At a small-town remodel gig, we rushed painting trim in a closed room—coughs, itchy eyes, and a headache swept through the room before we hit lunch break. Not one of us wore a mask. That wake-up call made me dig into the product’s information sheet and, since then, nobody on our team skips goggles or open windows. It’s easy to downplay risk until the fumes start to build up.
The right response isn’t panic—it’s common sense. Move work outdoors or crack a few windows if the weather allows. At bigger sites, local exhaust fans work wonders to clear the air. Workers can protect skin with gloves and long sleeves. Splash goggles guard the eyes, especially if spills ever happen. Respirators rated for organic vapors get some use during big painting projects or when using solvents in poorly ventilated spots.
Just because something isn’t classified as a cancer risk doesn’t mean people should ignore smart habits. Companies owe teams more than simple compliance. Sharing up-to-date safety sheets and offering training on clean-up and first aid beats out any shortcut. People at home can skip most risks by reading the labels and respecting the warnings—skip the guesswork.
Safer paints and cleaners keep arriving on store shelves. Water-based options or “low VOC” products hit the same performance targets for most jobs around the house or shop. Sometimes nothing replaces a tough solvent, especially in heavy industry, but that doesn’t make ignoring risk acceptable. Companies testing and shifting to less toxic ingredients can cut down exposure for everyone.
There’s space to keep learning. Researchers at universities and industry labs push to uncover gaps in safety data that matter for everyday use. Faster, clearer warnings, and better education can shrink the risk, even for people not directly handling chemicals every day.
Dipropylene glycol monomethyl ether acetate stands out in industries like paints, coatings, electronics, and printing. It’s not a household name, but it finds its way into a lot of places. The moment anyone treats it like regular solvent, problems can start piling up. I’ve watched folks in shops treat it casually, only to lose an afternoon cleaning up spills, and worse, risking their own safety.
Storing this liquid means thinking ahead. Dipropylene glycol monomethyl ether acetate prefers a cool, dry spot away from sunlight and heat. Too much warmth cranks up evaporation and can even lead to hazardous conditions. Most manufacturers give a range of 15-30°C for a reason: it keeps the liquid stable, slow to break down, and easier on containers over time. Humidity kicks rust or corrosion into gear, so keeping moisture out really matters.
Containers matter just as much. Metal drums with secure, tight-fitting lids tend to hold up well, but newer, high-density polyethylene containers handle long-term storage just as well if checked for compatibility. Flimsy plastic or containers with questionable seals only court disaster, leading to leaks or vapor hazards. Ventilation in storage areas remains critical. If vapor lingers, headaches, dizziness, and even possible long-term health problems follow, according to CDC case studies.
Anyone working with dipropylene glycol monomethyl ether acetate gets a crash course in PPE. Gloves made from nitrile rubber keep the skin safe, and goggles or face shields block any stray splashes. Workers in my field also stick to cotton or flame-retardant clothing—synthetics don’t fare well if the solvent contacts skin or starts a fire.
Poor labeling or assuming everyone nearby knows what’s in a tank creates real dangers. I’ve seen supervisors run through chemical handling routines with new workers every month, never once treating it like a background task. Labels in plain English, safety data available nearby, and regular reminders reduce accidents and confusion, especially in high-turnover plants.
Small spills happen everywhere. Letting them soak into floors or rags is a big mistake. In industrial settings, non-combustible absorbents and spark-proof tools work best. Sealed waste containers—clearly marked and regularly emptied—prevent buildup, and nobody wants to breathe in fumes from last week’s cleanup. Local laws usually set strict limits on how much can get dumped in drains or trash, so people rely on licensed hazardous waste companies for disposal. Fines don’t come cheap, and the environmental cost can run higher.
Stories from neglected warehouses fill industry forums. Containers bulge, leaks spark fires, and lost inventory creates emergency headaches. Accidents traced back to simple steps—poor housekeeping, ignored safety sheets, skipped refreshers. After seeing a small fire in a neighbor’s plant settle into the local news, I learned just how quick things can change.
Success here looks ordinary: regular checks for leaks, clear aisles, simple signage, reminder training, and up-to-date inventory sheets. Crosschecking with updated safety data sheets and acting on small problems early sidesteps disaster. For businesses, investing extra in proper storage gear and training brings real savings—in dollars and in peace of mind. No cutting corners. Good habits around storage and handling of dipropylene glycol monomethyl ether acetate save more than just trouble: they guard health, budgets, and reputations.
Dipropylene Glycol Monomethyl Ether Acetate shows up in plenty of workplaces. I've spotted it in coatings, cleaners, inks, and sometimes even in labs tucked away on campus. As a solvent, it’s handy but it can pose issues if someone treats it as just another liquid waste. The safety data sheets list it with warnings about affecting air quality and being tough on aquatic life. Tossing it in the drain or letting it evaporate out back won’t cut it.
People sometimes ask why anyone should care about an industrial solvent trickling into a wastewater system. Public water sources aren’t designed to filter out every chemical, especially those with complex molecules or slow breakdown rates. Exposure brings possible headaches and irritation to workers, but the toll on wildlife shows up farther down the line. I’ve read cases where improper solvent handling led to fish die-offs and polluted groundwater.
Regulatory tags like RCRA class certain solvents as hazardous, and local ordinances push for strict controls on chemical disposal. In my first job, the paint shop kept all used solvents in labeled drums. Weekly pickups by certified waste handlers were the norm. It costs more than dumping things down the sink, but cleaning up a chemical spill or a contaminated well can drain budgets and trust much faster.
Collect Dipropylene Glycol Monomethyl Ether Acetate in sturdy, labeled containers that handle spills. I always looked for leak-proof seals and made sure the labels included both the chemical name and the main hazard signals, like flammable or toxic. Keeping different types of chemicals separate helps avoid dangerous mixing that can create even bigger hazards.
Hazardous waste companies specialize in neutralizing or recycling solvents like this one. They show up with the right paperwork, understand the local law, and guarantee the waste lands in a regulated facility. Sometimes, parts of the solvent get recovered and reused in industry, lowering the total impact.
Companies do best when everyone handling chemicals knows the right steps. In my experience, regular workshops that use real examples make a difference. People work better as a team when they trust the process and see leadership backing proper solvent disposal.
On rare occasions, I met folks who found Dipropylene Glycol Monomethyl Ether Acetate in a home workshop. Instead of flushing it, they checked for household hazardous waste drop-off days. City and county programs take these chemicals and keep them out of rivers and the air.
As more products go green, choices for less hazardous alternatives grow. That will help future workers and neighborhoods. Today, the core priority stands clear: smart handling and responsible disposal protect health, water, and reputation. Each bottle handed off to a waste hauler means problems don’t slide downstream.
Dipropylene glycol monomethyl ether acetate looks like a clear, colorless liquid. It flows easily and gives off a faint, mild odor, nowhere near as overwhelming as strong solvents like toluene or acetone. The flash point sits at about 75°C, so it doesn’t catch fire at the slightest spark, but a hot enough source could still start trouble. It's not a substance that evaporates quickly. The low vapor pressure—around 0.2 mmHg at room temperature—means that it hangs around for a while once exposed to the air. This quality lets painters and industrial workers have more time to work before the coating dries out.
The boiling point, at roughly 220°C, lands this chemical in the high-boiling group. Pouring it into a beaker or drum, you’ll find it mixes well with other organic solvents like alcohols and esters, but it won’t play nice with water beyond a certain level. That behavior comes from its structure, which gives it some polarity, but not enough to be fully comfortable in water.
The name alone signals two things—a glycol backbone and an ester group. This structure gives it modest reactivity. In day-to-day use, the solvent keeps a stable profile under neutral conditions. Exposing it to strong bases or acids can break up the ester group. For example, if caustic soda or hydrochloric acid ends up in the same line, hydrolysis can split the molecule into its starting parts. Companies that move large batches of coatings watch carefully to prevent cross-chemical mishaps for this reason.
Flammability risk grows in confined spaces with poor ventilation. Inhalation at high levels may cause headaches and nausea, so shops and factories rely on exhaust fans, open doors, or protective masks, especially during hotter and busier shifts. The chemical is not classified as a major health or environmental hazard in the same league as strong solvents or chlorinated hydrocarbons, but mishandling it can irritate the eyes, skin, or lungs. Workers usually learn to manage the risks with gloves, goggles, and good workflows.
Paint makers, electronics manufacturers, and ink shops choose this solvent for its balance. It helps dissolve both polar and non-polar resins, so blends lay down smoothly and dry at a controlled pace. Fast-evaporating solvents can leave behind streaks or bubbles. Dipropylene glycol monomethyl ether acetate stretches the working window—a crucial factor for car refinishers or artists who need time to fix imperfections.
Comparing it to similar compounds, like propylene glycol monomethyl ether acetate, engineers notice minor differences in evaporation, solvency, or odor. Subtle tweaks in chemistry produce real-world shifts in safety limits and equipment needs. Tight regulations demand that shops track every drum, train new hires, and limit exposure to protect health.
Solvent users often look for options with a smaller environmental footprint. This chemical, while less threatening than older hydrocarbons, doesn’t break down overnight if spilled. Cleanup guidelines call for quick containment, proper storage, and avoiding storm drains. Companies invest in better ventilation, leak monitoring, and staff training to prevent most incidents before they start. Some industries test bio-based alternatives, but few match the same drying curve or solubility profile. Ongoing partnerships between chemists, regulators, and the end-users keep pushing for safer blends and greener processes, inching the industry forward.