Back in the 1940s, the world’s chemical laboratories saw a period of growth driven by the quest for better solvents. Dipropylene Glycol Methyl Ether (DPM) emerged through this innovation, offering something unique compared to traditional glycols. Chemical engineers were searching for less volatile, safer alternatives to older solvents that produced harsh fumes. The push for safer working conditions, especially post-war, made DPM a suitable candidate for companies reformulating cleaning products and paints. Early industrial tests highlighted DPM’s lower evaporation rate, which in turn set a new standard for workplace safety. Over decades, production scaled up as more sectors realized its benefits in both manufacture and end use.
DPM’s clear, practically odorless liquid form sets it apart from harsher solvents. Developed mostly for use as a coupling agent or solvent, DPM bridges the needs of industries looking for compatibility between water-based and oil-based components. Unlike some of the chemicals classified under glycol ethers, DPM won’t cause sharp changes in pH levels, and that stability has helped it gain acceptance for cleaning, coatings, and electronics manufacturing. Paint formulators often turn to it because it spreads evenly, carrying pigment through and allowing color to hold fast, even after years of exposure. In labs, DPM often outperforms older solutions that tend to have higher toxicity profiles.
DPM sits at a boiling point near 190°C, which means it lingers on surfaces longer than fast-drying solvents. Water solubility remains an important trait, so it’s easy to rinse from equipment or surfaces. It weighs in at about 0.95 grams per cubic centimeter—dense enough for substantial cleaning, yet light enough for easy blending. DPM’s vapor pressure ranks as low, so it does not evaporate too quickly at room temperature. Acidity runs close to neutral, a key advantage in formulations for paints or cleaners that shouldn’t irritate skin or corrode metals. This property profile shows why so many manufacturers keep it in their toolkit.
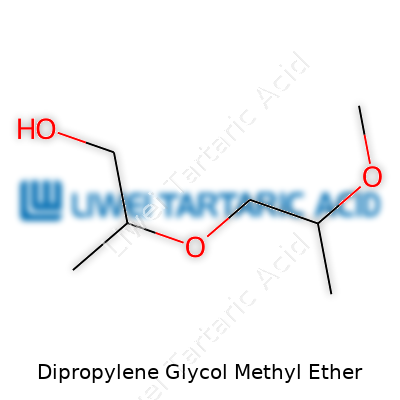
Chemically, DPM carries the formula C7H16O3 and generally meets purity levels above 99% for industrial applications. Material Safety Data Sheets (MSDS) often point out its flash point of about 75°C. You’ll spot DPM listed under CAS Number 34590-94-8. GHS-compliant labeling marks will warn users about eye and respiratory irritation if misused. Proper labeling not only helps meet regulatory standards, it also signals responsible manufacturing. Companies maintaining ISO 9001 certification closely monitor purity and batch consistency. Accuracy here protects both downstream users and employees who handle bulk shipments.
The backbone for DPM production comes from reacting propylene oxide with methanol under controlled conditions. Catalysts ensure the process pushes toward dipropylene glycol intermediates. Heat management plays a vital role. Too high a temperature and unwanted byproducts leak in, creating waste. Too low, and reaction rates drag out, driving up costs. Over the years, advances in reactor design and catalytic control cut down on emissions, a real win for local communities and plant workers. Synthetic routes now incorporate byproduct reuse, trimming both the environmental footprint and raw material bills. Today, chemical plants rely on refined distillation processes to pull out top-grade DPM, bottling it up for use across continents.
Chemical engineers have put DPM through rigorous testing to reveal its reactivity. DPM resists oxidation under standard conditions, so cleaner manufacturers use it to stabilize waterborne formulas. It stands up well in both acidic and alkaline mixes. In advanced settings, scientists tweak DPM into mono- or tri-derivatives, each with slightly adjusted evaporation rates or solubility. In some cases, blending with other glycol ethers expands its application range, tailoring performance for electronics cleaning or ink solubilization. Because DPM seldom reacts with plastics or sealants, it avoids damaging production lines—a huge plus in modern, automated factories where downtime cuts hard into profits.
In the chemical trade, you might run into DPM as Dipropylene Glycol Monomethyl Ether, DPGME, or Dowanol DPM. Industry catalogs sometimes label it as 1-(2-Methoxypropoxy)-2-propanol. These alternate names reflect differences in source or manufacturer, but all point to the core product. Some suppliers market modifiers or blends using DPM as a base, tucking in their own trademarks. Keeping track of these synonyms turns critical for compliance managers who might be handling multiple products labeled differently, but essentially identical in composition. That clarity helps avoid accidental double dosing in sensitive applications, such as flavoring production or high-precision coatings.
Working in a shop that uses DPM means understanding the right safety procedures. Direct skin contact may cause mild irritation. Long-term inhalation at high concentrations poses respiratory risks, confirmed in animal studies. Personal protective equipment—gloves, goggles, and, if splashes are likely, face shields—goes a long way. Ventilation stands as the first line of defense, with most industrial users installing local exhaust or dilution fans. Emergency protocols require spill kits rated for solvents and regular employee training. In Europe and North America, national agencies enforce workplace exposure limits to keep workers safe. Years in field service have shown that clear signage, up-to-date MSDS access, and regular audits help cut accident rates.
DPM touches countless industries, and experience dealing with paint shops and electronics assembly lines shows its value firsthand. In paints and coatings, DPM keeps pigments flowing evenly so no streaks appear. Manufacturers of cleaning products use it to lift stubborn soils from industrial machinery. It dissolves inks for printers that demand crisp, smudge-proof output—important when print quality means the difference between zero returns and high complaint rates. In electronics, DPM cleans delicate circuit boards without corroding tiny connectors. Personal care companies sometimes turn to DPM for its gentle solvency in hair styling products, leaving little odor behind.
Research teams continue to hunt for ways to boost safety and lower emissions from DPM during production and disposal. Current studies examine the use of renewable feedstocks, aiming to reduce reliance on fossil-based propylene oxide. Green chemistry labs explore enzymes and biological catalysts as replacements for traditional acid catalysts, risking fewer waste products. I’ve seen collaborative projects between chemical plants and universities develop new tests to detect trace impurities faster, ensuring batches meet tight specifications. The push to develop DPM derivatives with reduced toxicity keeps getting stronger each year, with some startups reporting promising results from their pilot lines.
Scientists have extensively tested DPM for short- and long-term effects in both animals and people. Most studies rate DPM as low to moderate in toxicity, but they do not recommend ingestion or undiluted skin exposure. Evidence shows prolonged inhalation of high vapor concentrations can lead to headaches or mild nervous system effects. Workplace studies consistently call for robust ventilation and avoidance of open containers indoors. Community monitoring near chemical plants indicates emissions rarely reach harmful levels, thanks to stricter environmental rules. Environmental toxicology work aims to better understand how DPM breaks down in waterways and soil, shaping disposal policies for years to come.
Looking forward, DPM’s growth tracks society’s twin demands: safer products and cleaner manufacturing footprints. New environmental rules favor solvents that don’t stick around in the air for long. DPM’s low volatility gives it an edge as local and global standards tighten. As renewable chemistry takes hold, expect suppliers to roll out “greener” DPM grades derived from bio-based feedstocks, opening new markets among companies branded as sustainable. Startups and larger manufacturers alike search for DPM alternatives with lower toxicity and even better performance, a challenge that drives today’s chemical R&D investments. With the global supply chain shifting, companies that invest in cleaner, smarter DPM production will likely set the pace across solvents, coatings, cleaning agents, and precision manufacturing sectors.
People rarely think about the chemicals that help keep surfaces clean, paints smooth, or their workplace free of harsh odors. Dipropylene glycol methyl ether—sometimes shortened to DPGME—steps into these moments quietly but leaves a real mark. I remember working in a print shop and catching that faint, sweet smell in the air. Later, I learned it came from the press cleaners and inks, all relying on this one solvent.
Solvents act as the backbone for many products, keeping substances mixed and easy to use. In cleaning products, DPGME goes into glass cleaners, floor scrubs and industrial degreasers. It cuts stubborn grime and evaporates slowly, leaving more time to wipe stubborn marks. Long before fancy eco-labels crowded store shelves, janitors swore by cleaners with DPGME for removing smudges that nothing else would touch.
In coatings and paints, this solvent helps colors spread evenly. I’ve watched a painter roll a fresh coat along a wall, no streaks or bubbles, because the can listed DPGME as a key ingredient. Professional painters keep returning to brands that trust this solvent, because it balances performance with a lower odor that doesn’t drive people outside for air.
The print industry relies on chemicals that keep ink flowing fast and smooth. DPGME helps ink stay workable longer, which matters for anyone who’s wrangled dried-up ink rollers or watched sharp designs turn muddy. It’s all tied to how this solvent manages evaporation, slowing things down just enough. Factories, artists, and anyone who’s silkscreened a t-shirt rely on that little window of work time.
On the factory floor or shipping dock, adhesives use DPGME to spread without clumps. Strong, clear, and easy to handle—these glues hold together packaging, electronics, and building materials. Years ago, while helping a friend refinish furniture, the adhesive recommended for laminating desktop edges contained DPGME. Both the instructions and my own nose confirmed it was friendlier to work with than other strong-smelling options.
No chemical comes without risk. Safety sheets spell out what DPGME will do if splashed or inhaled. Compared with harsher solvents, DPGME usually earns a lower toxicity score and releases less vapor into the room. For workers, that translates to fewer headaches and less irritation on long shifts. Studies from government agencies, like the US Environmental Protection Agency, have collected data over the years that show this solvent breaks down in the environment faster than some alternatives. Yet, care makes sense: gloves, eye protection, and proper ventilation all matter. Product labels and regulations underline that point.
Moving forward, the conversation shouldn’t only focus on what DPGME can do, but how it fits into the bigger picture. Companies now look for ways to lower chemical loads and recycle air inside buildings. Researchers keep searching for solvents with even lighter environmental footprints. Trust builds through transparency about ingredients. Choosing safer options means both businesses and consumers share responsibility—asking questions, reading labels, and watching for changes in regulations or best practices.
Pick up a bottle of glass cleaner or fresh paint, take a glance at the back label, and you might spot an ingredient called Dipropylene Glycol Methyl Ether (DPGME). It hides under a chemical name, but it plays a role in thousands of homes and workshops. Its main job comes down to dissolving other substances and making them easy to spray or wipe on smooth. It also comes up in inks, floor finishes, and even electronics manufacture. The American Chemistry Council and toxicologists who study workplace exposures pay constant attention to how these solvents get used so frequently.
Many workers share space with DPGME all day—those who fill paint drums, pack cleaning supplies, or handle degreasers. Safety data sheets flag it as a low-to-moderate toxicity chemical. Touch too much, inhale heavy fumes in a closed room, or spill it on your skin every shift and some risk builds. Common side effects pop up as light headaches, mild skin irritation, or tingling fingers if the chemical lingers on hands. You’d need to have gallons of it dumped on your arms before real burns or liver damage show up, based on reports from industrial medicine researchers. The National Institute for Occupational Safety and Health (NIOSH) sets exposure limits, using animal studies and medical findings, to make sure workplace air stays clear enough to keep people safe. That limit stands at about 100 parts per million over an eight-hour shift.
Chemicals that clean well sometimes grab headlines for their persistence or toxic leftovers. DPGME breaks down pretty fast. Sunlight, air, and bacteria in soil break the molecule apart, so it doesn’t stick around in rivers or the crops farmers raise nearby. It won’t build up in fish or drift far on the wind. Still, draining gallons of anything down city pipes rarely ends well for wildlife. The U.S. EPA studies these runoff concerns closely and puts out clear recommendations for handling and disposal.
People want real answers for chemical safety, not ten pages of chemical formulas. Clinical studies with volunteers and long-term worksite monitoring from Europe and North America both show DPGME doesn’t cause cancer or genetic mutations when used with reasonable care. Using gloves, washing up with soap, and airing out the workspace keep trouble away. The Centers for Disease Control and Prevention (CDC) points out that workers already exposed every day show little sign of chronic harm, provided safety guidance gets followed.
Daily experience says, don’t act like it’s water. Always use gloves, especially if a job gets your hands wet or greasy. Keep a window open or a fan blowing when spraying anything indoors. If cleaning up a spill, grab paper towels or rags and toss them in the trash once dry. Don’t pour leftovers down the sink or toilet. City waste collection points or hazardous waste days exist for a reason. Education from supervisors, direct warnings on bottles, and health and safety boards all push for smarter handling, and it pays off.
You can keep a house or workplace tidy without putting anyone in unnecessary danger. Every chemical requires respect, but DPGME has built its reputation by not leaving much trace behind. Keep safety habits sharp, and this solvent stays on the list of low-risk household helpers rather than troublemakers.
Dipropylene Glycol Methyl Ether, often shortened to DPGME, finds plenty of use across paints, coatings, and cleaning fluids. It seems safe at first glance; the liquid carries only a faint odor, and spills don’t leap out as threatening right away. Yet working with solvents like this brings real risks. In my years around industrial warehouses and chemical storage, one thing stands out: assumptions create accidents. Many overlook DPGME’s slow evaporation rate or think it’s harmless because it doesn’t burn instantly like gasoline. Experience teaches the opposite—storing and handling chemicals always deserves real attention.
DPGME containers sit safest in cool, well-ventilated spots. Temperatures over 40°C push containers toward swelling and sometimes even rupture. I’ve seen colleagues learn the hard way—one hot storeroom leads to sticky residue on drums, leaks, and that unmistakable whiff of solvent. Fire risk isn’t dramatic compared to more volatile chemicals, but that’s no excuse to ignore the basics. Keep storage away from open flames and sparks. Static electricity lingers as a constant threat; a small charge can ignite vapors hovering in a warm, confined corner. Bonding and grounding storage tanks isn’t bureaucratic overkill—I've watched a simple discharge travel across a drum’s rim before anyone noticed.
Avoid stacking barrels too high. Weight crushes the lower drums, rupturing seals. That lesson stuck after helping clean up a sticky puddle from three broken drums. Store DPGME in containers made from materials like stainless steel or certain plastics; incompatible linings, especially zinc or aluminum, create nasty reactions that compromise product quality. Always ask suppliers for compatibility sheets and check existing storage racks for corrosion or punctures after spills.
Pouring or transferring DPGME calls for focus and training. Even without an aggressive smell, fumes can affect the nervous system, causing headaches or dizziness. A splash on the skin seems mild at first—until irritation sets in or a rash appears after repeated contact. Wearing chemical-resistant gloves, long sleeves, and splash goggles keeps minor incidents from turning worse. Simple latex gloves might break down; nitrile or neoprene stand up better over time. Respiratory protection doesn’t always feel critical with DPGME, but in small rooms or during cleaning jobs, a properly fitted mask blocks airborne solvent mist. Eye washing stations must stay nearby, ready for accidental spray or splash.
No one expects spills, but plenty of workers end up mopping up more than they intended. Fast response comes down to preparation. Absorbent pads, sand, or inert materials stop spread without reacting with the chemical. Never wash DPGME down the drain—local water systems and wildlife suffer for it later. Buildup in sewers increases explosion hazards. I stress the importance of labeling: Drums and shifted containers without clear labels lead to decisions made on guesswork, and guesswork gets people hurt.
Training drives safety culture. Every staff member handling or storing DPGME should walk through spill response and fire drills; stories of “old hands” skipping these steps rarely end well. Regular checks on all storage areas—dates, seals, temperatures—catch small issues before they turn into emergencies.
Deep understanding and ongoing respect for what chemicals offer and what risks they carry help keep workplaces safe. Process improvements—ventilation upgrades, better PPE, tighter procedures—add real protection. Sharing lessons learned, both successes and near-misses, builds knowledge that keeps people safer, and keeps business running without costly, hazardous interruptions.
Dipropylene glycol methyl ether shows up in cleaning products, paints, coatings, and even electronics manufacturing. Many people see the word “glycol” and think of antifreeze, but this compound steps into everyday life far beyond just coolants. With so many companies talking about sustainability, it’s easy to wonder if this chemical truly lines up with green goals or not.
Factories create dipropylene glycol methyl ether by reacting propylene oxide with methanol. The process usually doesn’t create huge volumes of dangerous byproducts. Technicians work in controlled conditions with well-established safety steps. Compared to older solvents loaded with heavy metals or chlorine, this one seems tame. Air and water breakdown does eventually occur, but the road to complete decomposition takes some time.
In water, microorganisms can digest this compound, transforming it into smaller, less harmful molecules. The Environmental Protection Agency has checked the pace of breakdown. Most estimates say it clears out of streams or soil in a few days to a couple of weeks under the right temperature and conditions. That’s quick by solvent standards. Still, at large concentrations near factories, the story changes. Overloading the system can stress out local bacteria, and aquatic life suffers. For example, when a spill hits a small creek, the oxygen drop leaves fish gasping. These accidents, while rare, serve as a reminder that even “mild” chemicals carry risks.
Dipropylene glycol methyl ether doesn’t build up in tissues or the food chain. It doesn’t act like mercury or PCBs that keep coming back through fish or birds. Even so, direct handling matters. Workers complain of headaches or nausea if ventilation slips. Regulations place exposure limits for a reason. PPE and careful ventilation matter in the workplace—not just to meet rules, but also to keep workers healthy.
Switching from stronger, more toxic solvents such as toluene or xylene brought down air emissions in the paint and coatings industry. Dipropylene glycol methyl ether helped lower workplace illnesses and even cut hazardous smog-forming gases. Yet, calling it fully “green” stretches the facts. It still leaches into groundwater near careless disposal sites, and improper burning sends fumes into the air.
Some new companies test plant-based alternatives like D-limonene or ethyl lactate. These break down even faster in soil and water. They smell less harsh and support farm economies, not oil fields. The catch comes in price and performance. Not every cleaner wants to spend extra dollars on a greener bottle. Customers judge with their wallets, and stricter safety laws take time to catch up.
People living near manufacturing zones deserve clear answers. Companies can run regular water tests downstream and report honestly. Simple changes—upgraded spill barriers, better staff training, choosing smaller batch sizes—do more for safety than recycling buzzwords. If cities invest in hazardous waste drop-off sites, families can throw out paint leftovers and cleaning fluids responsibly. Regulators can tighten rules around disposal, nudge businesses toward plant-based solvents, and reward smart design. At home, supporting brands that publish full lists of ingredients and explain their choices makes a surprisingly big impact.
Each purchase or policy tweaks the balance. For now, dipropylene glycol methyl ether rates safer than many old-school solvents, but calling it harmless does not match the evidence. Pushing further toward green chemistry means staying honest about tradeoffs, not just chasing the next trendy chemical name.
Spending enough time talking to chemists or walking through a factory floor, the name Dipropylene Glycol Methyl Ether (DPM) comes up more than people might expect. I’ve seen its boxy containers stacked near paint-mixing stations, piled near industrial cleaning supplies, and even mentioned offhand in printer repair shops. People who don’t work in chemical processing might never notice it, but in a lot of workplaces, this solvent quietly helps make products run smoother, cleaner, or even just look good.
The first spot DPM regularly lands is in the paint and coatings trade. Ask a technical rep from a paint company, and they’ll tell you it helps control how quickly a formula dries. Fast evaporation leads to streaks or uneven finishes, but too slow, and nobody wants to wait for a wall to dry. DPM offers a steady medium pace. It helps pigments and resins stay mixed during storage. This role matters not just for wall paint, but automotive coatings, industrial primers, and packaging inks. Printers appreciate that it slows down ink drying on the press, reducing clogged nozzles and wasted runs. Headaches on those big jobs drop when DPM helps keep equipment humming and colors consistent.
DPM’s job doesn’t stop with colors. I remember getting a tour of a janitorial supply company and hearing how DPM replaced harsher solvents in glass cleaners and degreasers. Strong solvents like ammonia and toluene might strip away grime, but they also push workers to wear more protective gear or work with better ventilation. DPM cuts grease and dries with less residue than old-school options. Its low toxicity profile means people working with it face less risk of headaches or skin irritation. For companies juggling safety, cost, and performance, switching to DPM became an easy call.
While working with a client in the fragrance industry, I noticed DPM listed right after alcohol on the ingredient label for some body sprays and lotions. In personal care, the goal isn’t just mixing fragrance well, but also making sure that the scent releases steadily and doesn’t disappear too fast. DPM helps dilute concentrated perfume oils, stabilizing scents and providing a comfortable feeling on skin. Using high-purity grades, companies offer products that don’t cause irritation or leave sticky residue, making DPM nearly invisible but crucial in daily routines.
The world behind circuit boards and microchips demands precise cleaning after assembly. DPM lands in the cleaning solutions that sweep flux and soldering pastes off delicate connections. Messy boards lead to short circuits or early failures, so manufacturers trust DPM for its low volatility and ability to lift residues without damaging seals or plastics. Its steady evaporation keeps things from drying too quickly, preventing spots or deposits. In a field where failure means lost time and money, that reliability matters.
Experience on factory floors and in product labs taught me that balancing performance, worker health, and environmental impact isn’t a one-time fix. DPM ends up the choice for so many industries because it threads that needle. Replacement options require serious investment and research. Pushes for greener and safer chemicals will keep changing recipes, but for now, practical results and workplace safety keep DPM’s reputation strong in paints, cleaners, personal care, and tech.