The story behind dipropylene glycol ethyl ether traces back to the ongoing search for safer and more efficient solvents, especially in the last century. Before the 1950s, industries stuck to options like mineral spirits or plain alcohols, both of which carried their own risks of toxicity and flammability. Chemists kept hunting for compounds able to do the hard work of dissolving stubborn resins, inks, or dirt, without endangering workers or wrecking finished goods. As polyether technology evolved, manufacturers realized that stringing a few propylene oxide units together, with a final attachment of an ethyl group, could produce a molecule smooth enough to blend in cleaning and coatings, yet gentle enough to lower hazard ratings. Businesses looking for alternatives to glycol ethers with stricter VOC (volatile organic compound) limits, especially in the US and Europe, started to favor dipropylene glycol ethyl ether, ushering it into busy factories and formulation labs.
Today, dipropylene glycol ethyl ether turns up in a dazzling range of industrial and consumer products. It dissolves grease in professional cleaning products, serves as a coupler in waterborne paints and coatings, and finds its way into inks, dyes, agricultural formulations, and even some personal care products. Most makers keep a steady supply of the material due to its versatility. The networking power of this glycol ether—made possible by both the ether and alcohol functional groups—lets it carry active ingredients through a solution without raising flammability or leaving strong odors.
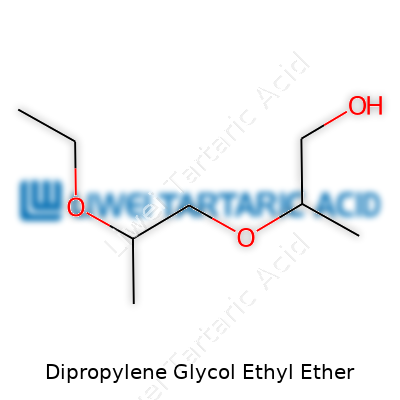
Talking about its appearance, dipropylene glycol ethyl ether usually shows up as a clear, low-viscosity liquid. There’s barely any color, and no strong scent gets in the way of its use in delicate formulations. Its boiling point typically lands around 200°C, with a freezing point far below water. It’s got a modest vapor pressure, which means it doesn’t easily evaporate at room temperature. The ethyl side chain hands it extra solubility in organic materials, while the glycol backbone lets it slide into water-based systems without fuss. Chemists looking at its molecular formula—C8H18O3—recognize the balance between polarity and nonpolarity, which explains why this solvent feels at home in both camps.
Producers sell dipropylene glycol ethyl ether in drums or totes, clearly stamped with lot numbers, purity data, and hazard symbols. Typical specifications list purity over 98%, water below 0.2%, and acidity kept near neutral to protect raw materials. Bulk handlers rely on UN shipping codes and GHS (Globally Harmonized System) compliant labels to mark out fire or health risks. Safety data sheets must keep up with regional regulations, flagging emergency contacts, exposure limits, and compatibility warnings. In factories, staff count on color-coded piping and frequent tank checks to steer clear of leaks or mishaps.
The journey to create dipropylene glycol ethyl ether usually starts with propylene oxide. Producers trigger a reaction called "etherification"—adding ethylene alcohols to drive the formation of a dipropylene glycol backbone. With a targeted alkoxylation step, ethyl groups join the mix under tightly controlled temperatures and pressures. Catalysts help route the reaction, pushing for a balance between mono, di, and tri-propylene glycol chains. Once the chemical dance winds down, vacuum distillation strips out any calcium, leftover reactant, or water, fine-tuning the quality before sending the product off to the bottling line.
Chemists treat dipropylene glycol ethyl ether as both a tool and a starting point. In cleaning blends, it nudges grease molecules apart without breaking down base materials. In paints, it helps other additives disperse evenly, limiting the need for harsher co-solvents. If extra performance matters, formulators can tweak the ratio of propylene units or tie on longer alkyl chains, shifting the solvent’s balance between speed and staying power. Modifications often chase after improved low-odor properties, lower flammability, or tighter VOC compliance. Research on industrial “green chemistry” keeps hunting for even cleaner synthesis steps, hoping to cut out by-products or reduce energy consumption.
Shopping around for dipropylene glycol ethyl ether, folks run into an alphabet soup of names—DPGEE, Dowanol DPM-E, or Propasol E, to name just a few. International agencies and chemical suppliers sometimes stick to CAS Registry Numbers—mainly 1569-17-1—to guarantee everyone talks about the same compound, no matter if the order form reads English, German, or Chinese. Industry catalogs show up with both the scientific mouthful and friendlier trade names, spelled out for paint shops, labs, and plant managers. Buyers learn to double-check documentation to steer clear of unexpected substitutions.
Nobody gets far with glycol ethers without thinking about safety standards and workplace habits. The US Occupational Safety and Health Administration offers clear exposure limits, echoing advice from Europe and parts of Asia. Personal protective equipment—gloves, goggles, and splash-resistant aprons—lands near the front of every safety guide. Ventilation fans and vapor scrubbers stick around plant lines to suck away any stray fumes. Staff who handle the product train on spill response, fire control, and first aid, practicing real-life drills to sharpen memory for emergencies. Regular air monitoring joins routine physicals for workers, catching problems before they get a chance to grow.
Dipropylene glycol ethyl ether’s reach stretches across paint factories, printing shops, agricultural chemical producers, electronics cleaning plants, and janitorial supply warehouses. It keeps graffiti-removal crews stocked with tough-yet-safe solvents. It breaks down stubborn residues in water-based adhesives that bond furniture and flooring. Print facilities, facing ever-sharper environmental rules, turn to DPGEE for inkjet printer cleaning solutions where fire codes and building ventilation matter almost as much as streak-free print runs. Agricultural chemical makers add it to pesticide sprays for better wetting and spreading action, aiming to reduce the need for stronger, more hazardous surfactants.
Research teams push dipropylene glycol ethyl ether in all sorts of directions. Formulators looking to extend product shelf life or widen compatibility tinker with blend ratios or try swapping out related glycol ethers. R&D teams at coatings companies track market signals—watching moves on VOC rules, green chemistry initiatives, and customer expectations about workplace safety. Startups and universities test out renewable feedstocks, such as plant-based propylene oxide, to lower the solvent’s carbon footprint. Testing covers not just performance in the final product, but also how the compound behaves over long storage or extreme temperatures. Green chemistry groups run pilot projects aiming to cut down on non-renewable sources and clean up water and energy use.
Clinical researchers and toxicologists keep a close eye on the safety profile of glycol ethers. Much of the published data draws from standardized animal studies, measuring skin irritation, eye contact, inhalation, and metabolic fate inside living systems. Collected evidence suggests that dipropylene glycol ethyl ether scores lower on acute toxicity compared to shorter-chain cousins like ethylene glycol ethers, though repeated exposure over long periods remains a subject of careful study. Regulators set exposure limits based on this research, inviting outside experts for peer reviews and updating guidance as new results roll in. Attention to breakdown products and metabolites helps spot far-off risks, and environmental scientists run fate-and-transport studies to check how much lands in rivers, soil, or air after disposal.
Looking forward, the journey for dipropylene glycol ethyl ether likely leans into stricter regulatory environments, pressure for biodegradable solutions, and faster production cycles across paint, printing, and cleaning supply markets. As more companies chase after smaller carbon footprints and lean manufacturing, demand for solvents that check both performance and sustainability boxes stands to rise. Ongoing breakthroughs in catalytic process design or crop-based feedstock technology could trim environmental costs even further. Buyers and manufacturers keep watching for a clearer link between fast-changing workplace rules and consumer preferences, knowing that the competitive edge rides not just on results in the lab but on how a solvent stands up to shifting global standards.
Dipropylene Glycol Ethyl Ether, often shortened to DPGEE, isn’t the sort of thing you see featured on a store shelf. Yet it quietly plays a role in plenty of everyday products. You probably come across its effects without noticing. For most people, the main connection to DPGEE shows up in cleaners and paints. Manufacturers use it to make liquids flow better and help ingredients stay mixed. Picture washing your kitchen counter with a streak-free cleaner—DPGEE probably helped get you there.
Having worked in facility maintenance, I saw firsthand how strong-smelling, streaky glass cleaners could cause headaches and sticky residue. Brands that switched to DPGEE a few years ago gave us cleaners that performed better, both in how they spread and how they dried. It didn’t hurt that complaints about lingering odor numbers dropped too. DPGEE isn’t just about convenience, though; it’s safer to handle than many older solvents.
People working with paints and coatings tend to prefer DPGEE for a simple reason—it does the job without drying out skin or releasing harsh fumes. Some solvents can irritate lungs and skin, but this chemical’s milder profile lets crews get work done without so many safety breaks. Recent safety studies back this up, showing low toxicity and very little risk under normal use. That’s a relief in trades where skin and lung exposure add up over decades.
As a glycol ether, DPGEE dissolves both oil and water-based substances. This versatility matters in products that need to clean a greasy stovetop or thin stubborn old paint. Switching older cleaners to greener chemicals sometimes made them less effective, but DPGEE bridged that gap. It helps products clean thoroughly without bringing back the harsher chemicals people were trying to leave behind.
DPGEE isn’t just about cleaning and paint. It shows up in printing inks, adhesives, and some specialty textiles. Printers use it to keep ink from drying out too fast or gumming up machinery. Furniture and automotive shops reach for adhesives that rely on DPGEE to bond tricky surfaces and cure without leaving brittle residue. It’s a workhorse, quietly giving everyday items a smoother finish or longer lifespan.
Because the chemical world moves fast, regulators keep a close eye on substances like DPGEE. Agencies such as the U.S. Environmental Protection Agency and European Chemicals Agency currently rank DPGEE among lower-hazard solvents. It breaks down fairly easily in the environment, and isn’t known for building up in living systems.
There’s still work that matters around DPGEE. Some consumer advocacy groups keep pressing for even safer, biodegradable alternatives, especially for use in homes with kids or those with respiratory illnesses. Chemists are always on the lookout for ways to make cleaning agents even gentler, finding ways to swap petrochemicals for plant-based sources. But the track record for DPGEE has shown it’s already a step up from many predecessors.
In my experience, switching to safer, reliable chemicals shows up not only in reduced health complaints but in less downtime for cleaning crews and painters. The bottom line: DPGEE keeps everyday products working better with fewer risks, and that means people can focus more on the work at hand and less on what might happen later.
Dipropylene Glycol Ethyl Ether often finds its way into household products, commercial cleaners, and sometimes skin-care items. Manufacturers use it because it helps dissolve ingredients, making products smoother and easier to apply. For years, regulatory bodies like the US Environmental Protection Agency and the European Chemicals Agency have set limits for safe exposure, especially for workers in industries that rely on chemicals every day.
Looking at the science, this chemical has a reputation for low toxicity. Acute studies on skin show minimal irritation in most cases. The Cosmetic Ingredient Review board says short-term skin exposure rarely causes issues for healthy adults. At the same time, European regulators demand warning labels for products exceeding certain concentrations, signaling caution over comfort.
Anyone who’s cleaned classrooms, worked in a hair salon, or fixed up cars will know these chemical names by memory. I’ve spoken to teachers and janitors who wipe down desks with strong cleaners. Their main concern is dried-out hands and sometimes red patches. Ask hairstylists—they wear gloves all day for a reason. Frequent contact, even with chemicals considered mild, piles up over the years. More people develop minor rashes or stinging if they’re working fast or skipping hand protection during a long day.
Watch out for higher concentrations. Patch tests and safety data agree: extended, direct contact with undiluted Dipropylene Glycol Ethyl Ether causes redness in some people. Most over-the-counter cleaners keep this chemical well below damaging levels, but mixing up makeshift solutions at home—trying to get that deep clean—raises your risk. All it takes is a small splash of the wrong concentration, and sensitive skin reacts.
Adults with eczema, dermatitis, or a long history of sensitive skin feel these effects sooner. Parents sometimes worry, too, seeing children play on freshly cleaned surfaces or helping with chores. Laying down some ground rules—like washing hands after use and.
Simple fixes make a difference. Gloves do their job better than sticking with bare hands, and regular breaks let your skin recover. Check the product label before starting anything that sounds harsh. If the instructions look vague, contact the manufacturer and request a full ingredient breakdown. A little investigation keeps surprises out of your daily routine.
Workplaces owe their employees more than just mandatory goggles. Routine safety training, clear signage, and wall-mounted dispensers filled with gentle moisturizers cut down on chronic skin irritation. Retailers have a role too, urging customers to follow recommended dilution ratios and giving honest advice about protective gear, especially for the “do-it-yourself” crowd.
The science doesn’t paint Dipropylene Glycol Ethyl Ether as a major villain, but every chemical deserves respect. Minor irritation, once in a while, might not matter much. Let it build up, or take shortcuts, and the damage starts to add up. There’s always a smarter way to do the job—read the label, keep your skin shielded, and speak up if any reaction starts. That’s how you work alongside chemicals without letting them cut into your health.
Dipropylene glycol ethyl ether has always drawn interest across various manufacturing sectors. Used in paints, coatings, cleaners, and inks, this solvent finds its way into daily production routines. Still, many overlook its storage and handling details, which matter just as much as the chemical’s performance. Years spent on factory floors teach you quickly: a small mistake can snowball. Spills, leaks, or accidental exposure not only carry safety risks but also economic setbacks.
A warehouse or workshop works best for storing dipropylene glycol ethyl ether when it’s cool and dry. Direct sunlight and heat sources shorten product life and raise safety concerns. Metal drums or specialty containers, always with tight-fitting lids, ensure this solvent stays protected from contaminants and moisture. Rooms with plenty of airflow lower inhalation risks during accidental vapor releases. High humidity creates its own problems, so well-maintained storage areas with regular inspections prevent corrosion and unwanted chemical reactions.
Storing this product away from oxidizers and acids matters more than some expect. Chemical incompatibility causes hazards that are easy to miss until something goes wrong. In one instance at a cleaning products plant, a forgotten open container sat next to bleach compounds, resulting in a costly, worrying cleanup. Labels help, but habits prove even more valuable—always check neighboring chemicals, especially in shared spaces.
Personal protective gear sounds simple, though plenty of workplaces treat it as an afterthought. Chemical-resistant gloves, goggles, and long sleeves make a real difference. Years of handling solvents reinforce the point that splashes or skin contact rarely give warning. Proper ventilation, through open doors or exhaust fans, cuts down on vapor buildup and lowers exposure over long shifts.
Transport involves as much care as storage. Use carts or hand trucks made for chemicals, not just whatever sits nearby. Spills during transport waste product and frustrate coworkers. Clean, dry tools and hands keep containers sealed tightly. Always check each drum or pail before moving it—damaged packaging belongs nowhere near production lines.
Nobody enjoys cleaning up chemical spills, but a quick response limits damage. Always keep absorbent materials on hand for leaks, and train staff to use them without hesitation. Seal waste in appropriate drums, label it, and schedule prompt pickup by qualified handlers. Drains and local water sources deserve protection, since small mistakes lead to bigger headaches down the road.
Some facilities try to cut corners by mixing waste chemicals, but that’s never worth the risk. Keeping logs of every waste movement pays off during audits and helps spot patterns before they become problems.
Reliable storage and mindful handling help companies run safer, smoother operations. Cutting corners usually costs more than the investment in proper materials and staff training. When regulations change—and they do—keeping up is much easier for those with clean records and systems already in place. Dipropylene glycol ethyl ether makes work easier for countless industries, but only with the right respect for safety and practical habits.
Everyday cleaning products use a blend of chemicals to tackle stains, dissolve dirt, and leave surfaces looking fresh. Among the many solvents out there, dipropylene glycol ethyl ether (DPGEE) stands out for its unique blend of strength and safety. After spending years tackling tough messes as a professional cleaner, and plenty of weekends testing different products around the house, I recognize the traits that make a solvent valuable to both cleaners and consumers.
DPGEE breaks down grease, oil, inks, and adhesives. In glass and hard surface cleaners, it cuts through fingerprints and smudges without leaving streaks. In floor strippers and degreasers, it helps dissolve residue that many water-based ingredients can’t touch alone.
The U.S. Environmental Protection Agency (EPA) and European regulators list DPGEE as low in toxicity. Acute hazards fall way below most industrial solvents—so you won’t see the same skin irritation or respiratory issues that often show up with harsher products. I’ve handled it with basic gloves and eye protection, and never experienced the headaches that come from solvents like toluene or xylene.
In large commercial kitchens and auto shops, DPGEE makes a big difference. It evaporates at a moderate rate, which gives cleaning formulas time to work into messes but doesn’t leave puddles or residues. A strong solvent action also means manufacturers don’t need to pile in high concentrations, keeping products safer for skin and mucous membranes.
DPGEE smells faintly sweet, much less “chemical” than older solvents. For folks with sensitive noses—or those working in tight indoor spaces—this is a relief. Homemade cleaners rarely deliver the streak-free results I’ve seen with DPGEE-based glass products. No foggy film, and no worrying about lingering fumes.
The big question for a lot of consumers: is it safe? Based on the research out there, DPGEE shows a low profile for both acute and chronic toxicity. That does not mean pouring gallons down the drain or leaving bottles within reach of kids. Safe storage, careful handling, and basic personal protective equipment still matter. Every cleaner has a responsibility to follow the labeling and look for trusted brands that disclose their formulas.
From an environmental angle, DPGEE doesn’t bioaccumulate, and studies show fast breakdown in soil and water. Wastewater treatment plants can break it down under normal conditions. The cleaner industry still needs to consider total pollution load, especially with large-scale use, but compared to “old school” solvents, DPGEE lowers the risk. The switch toward greener chemistry benefits from replacing more hazardous compounds with something like this ether.
Consumers keep asking for safer, more effective cleaning options. Makers of cleaning products have an obligation to make hazard and ingredient information clear. The spread of third-party certification systems, like Safer Choice from the EPA, helps people sort out marketing claims from reality.
Regulators should keep reviewing new safety data and set limits for chronic exposure, especially as formulations change. Tough messes demand strong chemistry, but there’s a big difference between strong and reckless. With DPGEE, we can clean smarter without trade-offs that put our health or environment at risk.
People in manufacturing and cleaning industries come across two similar-sounding chemicals: Dipropylene Glycol Ethyl Ether (DPGEE) and Dipropylene Glycol Methyl Ether (DPGME). Both play similar roles as solvents, but their differences turn out to have a real impact depending on what you make or clean.
DPGEE and DPGME have structural similarities, yet one simple shift — an ethyl group here, a methyl group there — changes their properties quite a bit. The ethyl ether has a slightly bigger molecular structure. This small tweak affects how well each solvent dissolves certain types of materials, how fast it evaporates, and how likely it is to mess with your nose if you use it indoors.
Walk into a printing plant, a paint workshop, or a cleaning supply warehouse, and you’ll see both names pop up. In coatings and paints, the methyl ether evaporates faster and gives off less odor. This trait matters for folks who don’t want to breathe in fumes or who want quick drying. The ethyl ether evaporates more slowly, which can keep surfaces workable longer but might bother some with its slightly stronger smell.
Cleaning crews often rely on DPGME’s fast evaporation for glass and hard-surface cleaners. It helps avoid streaks and residue. DPGEE finds fans in ink manufacturers because it keeps print heads and surfaces wet just long enough for perfect color transfer, especially in environments where quick drying can ruin print quality.
No one wants skin rashes or headaches at work. The methyl ether generally proves gentler on skin. It gives off a low odor and has shown only low toxicity in studies. The ethyl ether, with its higher boiling point and slower evaporation, tends to stick around in the air longer. Workers exposed regularly need solid ventilation to prevent any long-term effects.
Personal protective equipment like gloves and goggles still matter no matter which ether you pick. I’ve seen folks neglect these basics and deal with irritated skin or watery eyes later. Companies following OSHA guidelines keep problems from popping up. Keeping Material Safety Data Sheets on hand and holding short, direct safety briefings about handling and storage makes a real difference for teams on the ground.
The conversation about chemical emissions gets louder each year. Both glycol ethers fall under regulations for volatile organic compound (VOC) content, which can lead to smog and trouble with air quality. DPGME ranks better in terms of environmental profile since it leaves the air faster and breaks down more quickly outside a controlled environment. DPGEE’s longevity may work against air quality if you’re not careful with room airflow.
Switching to water-based or lower-VOC formulations often comes up in product development meetings. Small tweaks—like swapping out one glycol ether for another—give companies a nudge toward better indoor air. Yet, performance in real-world applications sometimes holds companies back from making the leap, especially if product quality takes a hit.
The best pick depends on what’s most important: faster drying, safer indoor air, or performance on tough jobs. Working directly with chemical suppliers, and running tests on small batches, helps dial in exactly how either DPGME or DPGEE fits into new products or cleaning solutions. Transparency about ingredients and their impact has become a big selling point with customers. It builds trust while nudging everyone toward a healthier workday and a less polluted neighborhood.