Dipropylene Glycol Diacrylate (DPGDA) tracks its roots back to the growth of the acrylate industry in the middle of the twentieth century, responding to rising demand for materials that could bring quick curing and high flexibility to coating and polymer applications. Giant leaps in polymer science during the 1950s and onwards prompted chemical companies to explore new monomers, and DPGDA stepped into the spotlight as an answer for manufacturers wrestling with both cost and performance constraints. I remember studying early patents while working in a university lab during the ‘90s, and it was clear even then how DPGDA offered a middle road between more brittle, fast-reacting acrylates and denser, tougher monomers. Looking back, DPGDA shifted industrial coatings away from purely solvent-based systems, giving a boost to energy-efficient radiation-curing technologies.
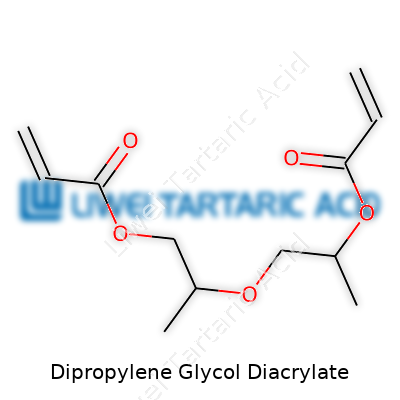
DPGDA falls into the family of multifunctional acrylates, carrying two reactive acrylate groups attached to a dipropylene glycol backbone. Commercially, suppliers offer it as a clear, low-viscosity liquid with a faint odor, packaged in steel drums or IBC totes. Specialty grades exist for electronics, dental, and inks markets, offering varying purity and stabilization options. Most pain points in the market center around the balance between reactivity and flexibility, and DPGDA lands squarely between highly reactive low-viscosity monomers and heavier, slower-cure analogs.
Dipropylene Glycol Diacrylate typically appears as a colorless or pale yellow liquid, with viscosity hovering between 10 and 15 mPa.s at 25°C, density around 1.02 g/cm³, and a boiling point in the range of 280°C under reduced pressure. With a flash point near 108°C, it doesn’t pose the same inherent hazards as many more volatile organics, but the double bonds in the acrylate groups remain highly reactive to air and light. DPGDA dissolves well in many organic solvents and enjoys a limited solubility in water, which makes it popular in formulations requiring quick mixing and uniform dispersion. Its low volatility and fast curing under UV or electron beam exposure place it firmly in the favorite’s circle for modern industrial coatings, adhesives, and photopolymers.
Suppliers usually guarantee DPGDA purity above 95%, with most grades containing less than 0.05% of free acrylic acid to minimize odor and toxicity. In practice, stabilizers such as methyl ether hydroquinone (MEHQ) get added to prevent premature polymerization—absolutely critical for facilities that store large volumes or transport DPGDA through hot climates. Labels must note UN numbers, hazard codes, and information on handling, especially the need for eye and skin protection. Some regions require added warnings about acrylate allergies, reflecting growing awareness in workplace health circles. Certificates of analysis (COA) accompany bulk shipments, permitting formulators to check critical quality benchmarks like color (APHA), inhibitor concentration, water content, and acidity before releasing into production.
DPGDA synthesis usually starts with dipropylene glycol and acrylic acid under strong acid catalysis, using standard esterification techniques. Producers often employ continuous removal of water to drive the reaction to completion, sometimes with vacuum or azeotropic distillation. After reaction, the mixture passes through neutralization, washing, and careful drying. Quality hinges on tightly controlling reaction temperature and purity of both glycol and acid, since impurities can trigger discoloration, off-odors, and gel formation during storage. Final purification must remove trace metals and catalyst residues, or risk gelling and premature curing in end-use environments. Stabilization rounds out the process, with MEHQ or related compounds mixed in to give DPGDA its signature shelf stability.
The two acrylate groups in DPGDA allow it to participate in rapid addition polymerization reactions, particularly under UV or electron beam irradiation. This reactivity underpins DPGDA’s use in crosslinking with other acrylates, creating tough yet flexible polymer networks that endure abrasion, moisture, and chemical exposure. In research settings, adding mono- or tri-functional acrylates tweaks final polymer hardness or flexibility. DPGDA can also serve as a base for further functionalization if end users require pendant groups for anchoring dyes, adhesion promoters, or anti-fungal agents. On a more practical front, it blends smoothly with photoinitiators and other oligomers, contributing to inkjet printing formulations or pressure-sensitive adhesives.
Industry professionals might recognize DPGDA under a few names: dipropylene glycol diacrylate, 2,2'-oxydi[propanol] diacrylate, or less commonly, bis-(2-acryloyloxypropyl) ether. Leading brands include Sartomer’s SR508, BASF’s Photomer 4028, and Allnex EBECRYL 225, each offering assorted stabilization packages for different markets. The push for REACH compliance and global safety standards means labels also spell out CAS number 57472-68-1, supporting traceability and registration across international supply chains.
Acrylate monomers, including DPGDA, demand strict respect for safety guidelines. Direct skin or eye contact sparks swift irritation; inhalation of vapors in poorly ventilated spaces creates risk of headache, nausea, or worse over long periods. I’ve seen unintended dermatitis outbreaks in print shops and dental labs skimping on gloves or eye shields. Good ventilation and nitrile gloves stop 99% of avoidable exposures, and safety data sheets lay out these precautions in fine detail. Facilities with bulk storage need to monitor inhibitor levels and keep oxygen exposure to a minimum, or risk runaway polymerization with dangerous heat release. Fire departments require flame-retardant storage and spill containment, as acrylates can flash when spilled near sparks or open flame. Annual safety training for operators, with case studies on real incidents, locks home the need for basic respect when handling these monomers.
In practice, DPGDA forms a backbone for industries ranging from automotive and flooring to high-resolution inkjet printing. UV-curable coatings built around DPGDA have shortened production cycles in electronics, furniture, and packaging thanks to their quick-drying properties and tough, glossy finishes that don’t yellow over time. In adhesives, it brings both strong initial tack and lasting flexibility, making it ideal for double-sided tapes and mounting films. Dental labs use DPGDA-based resins for impression trays and orthodontic molds, targeting accuracy and patient comfort without long cure times. Its role in 3D printing stands out, balancing viscosity and reactivity for desktop and commercial machines making prototypes to dental aligners. Unlike older, more brittle acrylates, DPGDA-based films hold up through repeated bending—an asset for flexible electronics and modern wearable tech.
Academic labs and corporate R&D outfits have probed every angle of DPGDA: changing the photoinitiators that trigger cure, blending it with bio-based monomers, or even tweaking the length of the glycol segment for greener credentials. Some key advances emerged in reducing shrinkage during curing—critical for dental and 3D printing uses, where shape fidelity means less post-processing. Material scientists look to improve both adhesion to tough plastics and abrasion resistance, especially for electronics and automotive coatings. Sustainable chemistry efforts have tested hybrid DPGDA blends using recycled glycerol or plant-based acrylic acid, seeking to trim the industry’s carbon footprint. Analytical chemists also target residual monomer detection in final cured films, to make coatings safer for touch and food-contact surfaces.
DPGDA’s toxicity profile sits between low-volatile glycols and much more reactive acrylates. Animal studies show moderate skin and eye irritation with direct contact, and light inhalation exposure triggers nose and throat irritation but rarely long-term harm if handled properly. Chronic effects remain low in well-ventilated workspaces using protective gear, but allergy risk still lingers: repeated exposure in sensitive workers can trigger chronic eczema or respiratory symptoms. Regulatory agencies hold exposure levels to strict limits—Germany, for example, sets workplace maximums and enforces real-time monitoring for high-volume operations. Recent work tracks environmental breakdown too; DPGDA biodegrades fairly well but can load aquatic environments if released in bulk. Communities near chemical plants track water and soil levels as a precaution, pushing formulators to lower waste and invest in better effluent treatment.
DPGDA stands in a strong position as the world pivots toward low-emission manufacturing and faster digital production methods. More sectors demand UV-curable coatings and adhesives with rapid throughput, fewer volatile compounds, and high lasting flexibility—exactly where DPGDA scores. Future blends with plant-derived reactants may broaden acceptance in eco-certification schemes. Additive manufacturing keeps growing, and DPGDA’s smooth behavior in photopolymer baths, low odor, and cure reliability suit both desktop inventors and industrial giants building next-generation wearables and medical gear. Yet challenges persist—reducing allergy risk and finding safer stabilization systems will keep safety professionals engaged for years. Long-term, expect new molecular variants with tailored reactivity and even sharper environmental performance, but for now, DPGDA keeps finding a home wherever tough, fast, and low-maintenance coatings, inks, or adhesives earn their keep.
Walk through any hardware store, peek under the hood at the mechanic’s garage, or check out someone’s DIY crafting setup, and you’re likely bumping into the results of dipropylene glycol diacrylate, even if it doesn’t get name-dropped. The stuff rarely gets much attention from the average person, but it plays a hidden part in things many of us use daily—especially if you spend time thinking about adhesives, inks, or that durable finish on your last home project.
My hands-on run-ins with dipropylene glycol diacrylate happened in a two-man print shop. Whether we needed quick-setting inks or durable coatings that didn’t crack under sun or rough handling, this compound was one of the building blocks. It’s a favorite in UV-curable applications, from commercial printing presses to the coating industry. Factories use it in floor sealants that can handle forklift traffic and in composite materials for electronic devices. In dental clinics and nail salons, it contributes to the flawless finish of dental composites and gel nails.
The big draw comes from its dual acrylate groups. These make it react well under ultraviolet light, forming strong bonds in moments. Shops can cure coatings or adhesives in seconds. What that means is better productivity for business and less downtime for repairs. For people who like their gadgets sleek and homes looking sharp, it translates to longer-lasting surfaces and finishes.
From direct experience—bonding, for example, an acrylic display for an indie artist—dipropylene glycol diacrylate stands out for its flexibility and strength after curing. Some resins become too brittle and crack if you drop them. With this stuff in the mix, the result tends to shrug off a few bumps. This resilience makes it attractive, not just for beauty products or packaging but for more demanding gear as well.
Another reason professionals reach for this chemical is its relatively low odor. During a summer ventilation nightmare at our print shop, switching to formulations with less offensive fumes made working conditions bearable. This means less irritation for workers—and fewer complaints.
No chemical is perfect. Some people develop skin sensitivity after frequent contact, especially in industries that use UV-cured adhesives and resins. Reports from workplace safety data point out that direct handling without proper gloves or ventilation raises those risks. It reminds me of getting a nasty rash once after skipping gloves during a rushed job—never again. Companies are aware and push for protective equipment and airtight UV light chambers. The right education at work makes a huge difference: people stay safer, and accidents drop.
Regulators, including the EPA and corresponding European agencies, track industrial use and recommend safe exposure levels. The drive for greener chemistry keeps pushing research toward alternative monomers. Some companies now test bio-based versions to keep performance high and toxicity lower. Until replacements catch up, the game revolves around handling and risk management: keep the focus on safety from workbenches to factory lines.
Dipropylene glycol diacrylate might seem faceless, buried in label fine print. Its effects touch lives in obvious and subtle ways—making things stronger, shinier, and quicker to dry. Knowing where it shows up, how to use it sensibly, and when to be cautious helps keep businesses running, protects hands on the job, and gives that lasting finish you notice every time you walk past a well-sealed floor.
In many labs and workshops, chemicals like Dipropylene Glycol Diacrylate (DPGDA) are on the shelf for good reasons. DPGDA makes inks, coatings, and adhesives perform well. It isn’t some mystery miracle—it’s a practical tool. Anyone who’s seen paint harden or used UV-cured nail products has probably seen the results. That’s the upside. On the flip side, DPGDA asks for respect. Based on years around similar acrylates, the rules stay the same: skin contact means risk, and inhalation isn’t an option.
No one expects a chemical like DPGDA to be gentle. Acrylates belong to a family of compounds known for causing skin irritation and allergic reactions. It doesn’t take much: even brief contact, especially repeated, can leave skin red or itchy or spark sensitivity that lingers for months. Those with asthma or sensitive lungs face a higher risk. A whiff of the fumes once or twice might not seem like much, but years of research show repeated exposure can bring headaches, dizziness, and even longer-term changes to the skin’s health. The European Chemicals Agency tags DPGDA as a skin and eye irritant and notes the risk of serious eye irritation.
Long sleeves, gloves, and goggles make for more than a good look; they’re basic protection. I’ve seen colleagues get lax, skip the gloves for “just a minute,” and end up visiting the occupational nurse hours later. Stores don’t provide this compound in a dropper bottle by accident—volume and control both matter. It doesn’t matter if you’re working in a lab or just crafting at home. Ventilation is just as important; acrylates don’t belong in the lungs, and a fan in the window isn’t enough. Safety data sheets show that DPGDA’s vapor creates problems quickly indoors.
Factories using DPGDA take precautions because past incidents raised alarms. Regulations reflect lessons learned in real workplaces, not just academic studies. Agencies in the U.S., Europe, and Asia call for proper labeling and storage. Training isn’t just a hoop to jump through—it beats time in urgent care. In my own experience, training brings confidence, not just caution. No one likes red tape, but safety gear and closed-loop systems make a difference day in and day out.
Curiosity drives people to look for less hazardous replacements. So far, acrylate-based chemicals like DPGDA keep their spot because nothing else balances cost, performance, and availability as well. That doesn’t excuse shortcuts. Companies and universities have begun to improve ventilation, offer hypoallergenic gloves, and swap out old fume hoods for new. This kind of step doesn’t make headlines, but it shapes safer workplaces. Public access to up-to-date safety data means less guesswork for everyone.
Anyone thinking about handling DPGDA should look beyond the promises on the package. The facts are clear. Safe work practices protect skin and health, and a moment of extra care can keep accidents off the calendar. That isn’t fear—it’s just knowing chemistry always demands respect. Experience proves it every day.
People who've spent time around industrial chemicals understand how little details can turn into big problems. Dipropylene Glycol Diacrylate, a clear liquid often used in coatings, adhesives, and photopolymer production, packs a lot of chemical activity into every drum. One careless mistake or lack of attention can set off a chain reaction that risks both health and investments. This is not just about ticking a compliance box. The reality, for staff and business owners alike, is a blend of protection, long-term costs, and community safety.
Exposure to Dipropylene Glycol Diacrylate can cause skin irritation, respiratory discomfort, and eye injury. Repeated or prolonged contact can lead to sensitization. Remaining unaware or ignoring these risks results in lost work hours and growing distrust among workers. That risk grows if the storage area is poorly planned. Records show that mishandling acrylate compounds can trigger not just staff complaints, but full-scale facility evacuations.
Factories and warehouses already juggle space, workflow, and energy bills. All too often, someone shoves chemicals into available corners. Dipropylene Glycol Diacrylate needs to stay in a tightly closed container, away from sunlight and direct heat sources. Temperatures should stay below 25°C (77°F). Heat speeds up polymerization, and if that happens inside a container, you've got pressure building fast, sometimes enough to burst drums or ignite vapors.
Ventilation ranks high. Vapors settle near floors and wouldn’t take long to catch fire with a stray spark. Fire codes recommend storage in flammable liquid cabinets or dedicated chemical rooms. Walls, floors, and shelves should resist spills and allow quick cleanup. Hazard labels on every container should remain legible, not faded or covered by tape—audits always catch those shortcuts.
Most warehouse teams learn about gloves and goggles quickly. Still, the number of skin complaints and accidental splashes each year shows where reminders help. Written procedures should detail not just what staff should wear, but who checks those supplies, and how spills or leaks get reported. Spill kits, eye wash stations, and fire extinguishers need refilling and testing on a set schedule—diligence saves lives here.
More often than not, unsafe habits start because someone is rushing or the right gear sits too far from the action. Assigning trained staff as chemical safety stewards makes a difference. Spot checks and open discussions about close calls show workers that their voices matter. Sharing near-miss stories, instead of hiding them, improves buy-in and keeps the memory fresh of why standards exist. Insurance claims data back up these efforts: businesses with strong chemical management cut incident rates in half within a year.
Regulations shift, but one thing doesn't: that moment in the routine where someone decides to do the right thing or just hope for luck. A blend of sensible planning, day-to-day vigilance, and open team culture sets a facility apart. Putting careful storage standards into practice means families worry less, operations stay smooth, and everyone goes home healthy.
Dipropylene glycol diacrylate pops up in a lot of modern manufacturing, from adhesives to paints and coatings to 3D printing resins. Its popularity comes from how well it lets things cure or set when exposed to light or heat. Anyone who spends time on a factory floor or in a workshop probably recognizes that sharp, sweet smell from acrylate compounds. That smell spells out a clear warning: this chemical doesn’t mix well with skin, lungs, or eyes.
My early years in plastics manufacturing taught me the hard way that a chemical like this can turn into a big problem in no time. A friend of mine once wiped up a small spill without gloves. He wound up with painful redness and a rash that did not go away quickly. These chemicals can seep in through the skin, and allergies or chemical burns creep up before you know it. This story repeats across labs, warehouses, and DIY workshops.
Studies from the National Institute for Occupational Safety and Health (NIOSH) connect acrylates with skin irritation, breathing problems, and even asthma after repeated exposure. The chemical industry does not leave this stuff unregulated for good reason. OSHA suggests eye protection, ventilators for people working with large volumes, and prompt washing if anything splashes onto skin. There’s a reason you see pictograms with big red warnings on every jug or drum.
A lot of accidents happen when workers get rushed or skip simple steps. If you handle dipropylene glycol diacrylate, start with protection: good gloves (nitrile or butyl rubber works best), safety goggles, and long sleeves. Don’t wear latex—they don’t block acrylates. Lab coats or aprons add another layer of protection.
Ventilation protects your lungs. Splashy jobs or open buckets should never happen in stuffy rooms. Most modern shops install local exhaust, but a box fan by a window beats nothing if you’re working at home. Open windows and wear a mask approved for organic vapors. Relying on luck or your body’s toughness will catch up with you.
Hand washing after handling the chemical gets more important than people realize. Water alone is not enough. Soap and water followed by a full rinse clears away residues. Never clean your hands with solvents—the stuff just drives chemicals deeper into your skin.
Good habits in labeling and storage make a difference. Mark every container. Keep drums and bottles far from food or drink. Leaks and spills find their way everywhere, so sit bottles on top of lined trays or spill mats. Take time to read the safety data sheet. It might come across dense, but knowing proper storage temperature, what to do in a spill, and emergency contact info makes all the difference.
With more factories and makers experimenting with light-cured plastics and rapid fabrication, it’s easy to overlook real-world hazards. Dipropylene glycol diacrylate is useful and effective, but meeting deadlines never justifies risky shortcuts. Management needs to invest in training and gear. Nobody wants the hospital bills or health problems that follow a careless mistake.
If companies and makers look out for each other—through safe handling, open conversations about risks, and following the lessons backed by research—serious accidents shrink. Everyone deserves a job where going home healthy is the baseline, not just good luck.
Dipropylene Glycol Diacrylate (DPGDA) finds its way into many workplaces—from print shops to electronics lines. This molecule gives flex to UV-curable inks, coatings, and adhesives. People think of chemicals as timeless, but shelf life comes from more than a date on the drum. Experience says chemicals change. Even those with stabilizers start picking up moisture, taking in a bit of light, or whiffing oxygen anytime a seal cracks. For DPGDA, the clock ticks even faster in shops that get busy and warm.
Most producers give DPGDA a shelf life of about one year. Not a magic number—just how long the company promises the product still works like the data sheet claims. This figure often assumes the stuff stays sealed, cool, and out of sunlight. Set a pail in direct summer sun, and clarity often fades before you even open it. Store the product near a loading bay with fluctuating air, and you’ll spot yellowing months before the stamped date. Moisture can sneak in and hydrolyze the acrylate groups, which leads to unexpected gelling or scum. Some factories rig up nitrogen blankets to push out air and seal drums tight; those drums outlast any half-used pail with a crimped lid.
Bad chemistry costs real money. Once DPGDA starts polymerizing in the container, it moves fast. One shop near me once ran batches that started curing mid-application—resulting in ten grand tossed because nobody checked batch age. Even a little breakdown creates hazing in clear coatings, or ruins adhesion in printed circuit boards. Customers notice, especially when products shipped one month start to fail after installation. That leads to steep warranty claims for something nobody spots until it’s too late.
You can smell degradation early. Sharp, acrid notes. If you see particulates, cloudiness, or the drum feels warm, chemistry’s already running. In my time working with coatings, test batches saved projects more times than I can count. Just a small panel before a full run shows how the raw material behaves—way better than reading numbers off a label. Labs with GC/MS tools can track breakdown, but it comes down to daily care.
Nobody controls the weather, but product care makes a big difference. Store drums below 25°C, away from heat and sun. Tightly seal everything—don’t trust the snap cap alone. Big users invest in dedicated storage rooms with temperature and humidity control. Keep logs so anything past six months gets checked before each run. For smaller outfits, buying only what’s needed for two quarters limits risk and cuts waste. Ask suppliers for stability data and don’t be shy about batch testing—especially for critical jobs that put reputation on the line.
Maintaining DPGDA quality means treating the drum’s shelf life like you would any food: take only what you need, keep the rest sealed, and go by smell and sight as much as by official expiration. It’s not about focusing on one container, but respecting the way chemistry keeps changing in real time. Transparency with customers and partners helps too—share shelf life practices, and problems become a shared effort instead of a hidden risk. In my experience, that mindset saves both money and trust over the long haul.