Dipropylene glycol came onto the industrial scene as chemists pushed for cleaner, less hazardous alternatives to classic glycols used in resins, plastics, and personal care. Folks in the 20th century didn't have access to today's streamlined purification or rigorous standards, so early versions often came mixed with odd impurities. That changed with tighter standards and improved distillation technology; the push for more reliable and less odorous glycols really kicked up with consumer product growth in the 1960s and 70s. My experience working with older formulators has shown a clear shift in attitude after more refined propylene oxide conversions started yielding cleaner, higher purity dipropylene glycol by the late 20th century.
This is a clear, practically odorless liquid found in bottles across different industries. The three common grades—industrial, fragrance, and polymer—pop up in everything from deodorants to brake fluid and hydraulic systems. Dipropylene glycol stands out due to its low toxicity and suitability for perfumes and cosmetics. Unlike many other glycols, it does not carry a sharp scent, which makes it work better when creating products meant to be gentle or pleasant. I’ve handled both technical and fragrance grades in lab and production settings; the main lesson: always check your spec sheets, because purity means everything in sensitive formulas.
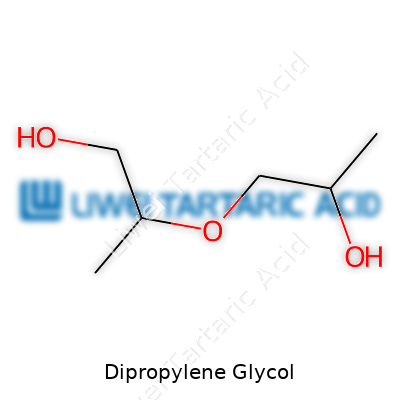
Boiling between 230-238 °C and weighing in with a density around 1.02 g/cm³, dipropylene glycol looks much like water but feels more viscous. It dissolves in water, alcohols, and some hydrocarbons. Chemically, it holds two alcohol functional groups and an ether linkage, which makes it chemically flexible. I’ve had to rework blends after underestimating its hygroscopic nature—the stuff will pull moisture right from the air, leaving mixtures off-spec unless properly sealed.
Manufacturers usually specify purity over 99.5% for industrial use, tighter for fragrance with minuscule impurities. Labels list CAS number (25265-71-8), batch number, and grade. I’ve seen that failing to mark the grade or letting barrels sit without tracking dates can easily cause mix-ups on production lines. This can create significant compliance risks, as end-product safety depends on correct raw material grades. On-site audits stress traceability from receiving dock to filling line.
Factories make dipropylene glycol by hydrating propylene oxide with water, often using a catalyst. This gives a mix of mono-, di-, and tripropylene glycols; fractional distillation then separates them out. The process uses precise control of reaction conditions: slight shifts raise unwanted co-products. Years of hands-on work taught me the importance of monitoring reactor pressure and temperature, as minor fluctuations can drive costs higher by boosting by-product yields and complicating purification.
With its dual alcohol groups, dipropylene glycol plays well with esterification and etherification reactions. These allow it to link up with acids to form esters used in plasticizers or react with isocyanates for specialty polyurethanes. I’ve worked on labs where tweaking the feed ratio makes a direct impact on the type and yield of downstream derivatives; every blend demands a new round of bench trials to nail down the right approach before upscale work.
Industry language rarely stays simple. Besides “dipropylene glycol,” the stuff sometimes goes by DPG, DPG diol, or “1,1′-oxybis(2-propanol).” Suppliers slap on trade names, which can get confusing when trying to source material for a specific application. Having managed procurement, I always encourage buyers to double-check technical datasheets and, if in doubt, request samples for in-house tests before locking into a new contract.
Compared to many industrial chemicals, dipropylene glycol doesn’t pose big acute hazards. It lacks significant volatility and flashes at high temperatures (over 124°C), so fire safety is less of a worry than for low-boiling solvents. Still, personal protective equipment helps avoid skin and eye contact, which can cause mild irritation after prolonged exposure. I remember one warehouse mishap where a leaking drum got overlooked, leading to slippery floors—the lesson: keep storage contained and check barrels for corrosion. OSHA standards and local guidelines dictate proper labeling and emergency response, including eyewash stations and training for safe handling.
This chemical’s reach runs wide. The biggest markets are cosmetics and fragrance, where pure grades shine as solvents that won’t steal the show with unwanted aromas. Personal care products rely on its mildness—think lotions, creams, and shaving gels. Dipropylene glycol also crops up in antifreeze, plasticizer production, and as humectant in tobacco products. HVAC technicians see it in heat transfer fluids. As new consumer expectations keep shifting toward lower-toxicity ingredients, demand patterns follow suit. My consulting work has shown that dipropylene glycol often replaces riskier glycols or solvents, thanks to a friendlier safety profile and steady pricing.
Current research has shifted toward greener production routes. Academic groups investigate catalysts supporting lower temperature reactions or biobased feedstocks for propylene oxide, aiming to lower the environmental footprint. Meanwhile, product development teams push for ultra-pure and specialty grades suited for food-contact and medical uses. My work on pilot plant trials demonstrated that tightening reaction controls and boosting recycling rates can cut waste, though keeping by-product glycols from drifting into the main product remains tricky. Collaboration with instrument suppliers to improve in-line purity testing sped up the learning curve and caught off-spec batches faster than old-fashioned wet lab tests.
Most modern data points to low acute and chronic toxicity. Oral LD50 values in rodents sit well above safety thresholds, though massive doses or long-term exposure might bring mild liver or kidney changes. A few years ago, toxicologists flagged concerns with skin irritation, mostly in high-exposure scenarios, like in poorly ventilated production halls. In modern workplaces practicing good hygiene and handling, the risks remain manageable. The European Chemicals Agency and US EPA both track long-term inhalation and ingestion studies, especially as new personal care products go to market. Responsible companies keep safety data sheets updated as soon as a new study surfaces, keeping the regulatory paperwork vigorous.
With global regulations tightening on solvent emissions and ingredient safety, demand for dipropylene glycol looks set to climb—not just in personal care, but also in technical markets like heat exchangers and lubricants. Consumer backlash against harsh preservatives and allergens has given manufacturers a push to adopt milder glycols. Innovations in process control keep squeezing operating costs, making high-purity grades more accessible to small and mid-sized players who used to get priced out. I’ve seen shifts on the ground as small-scale producers revisit their supply chains and invest in better training to keep up with stricter downstream requirements. Looking ahead, the blend of regulatory, technical, and consumer pressures will keep R&D teams busy pushing for cleaner, safer, more versatile dipropylene glycol lines. The days of generic, multi-use glycols are ending, replaced by tightly tailored grades matched for end-user needs—and that means opportunity for those willing to put in the legwork.
Dipropylene glycol shows up in places most folks don’t notice. It slips quietly into lotions, perfumes, cosmetic creams, and even cleaning products. My first brush with it came through a bottle of moisturizer that didn’t leave my skin feeling greasy. Skimming the ingredient list, I spotted a word I couldn’t pronounce—after a little research, I realized it played a big role in why that lotion felt so light.
Personal care products often rely on dipropylene glycol for its ability to dissolve and carry fragrance oils without irritating skin. If you’ve ever wondered why perfume mist feels cool and delicate instead of sticky, this ingredient helps deliver that sensation. Companies trust it because it keeps formulas stable and stops products from separating, which matters for anyone who expects consistent results each time they use their favorite lotion or spray.
Some folks worry about chemical names that sound unfamiliar, especially in products we use daily. Experts and regulatory agencies like the U.S. Food and Drug Administration keep a close eye on dipropylene glycol. According to the Cosmetic Ingredient Review, scientists have found it safe at the levels found in personal care items. They test for skin irritation and allergic reactions, and most reports say it’s unlikely to cause problems except in rare cases.
Still, using safe amounts matters, and open communication benefits everyone. I’ve learned from people with very sensitive skin or allergies—they watch labels for any ingredients that have given them trouble in the past. For manufacturers, clear labeling and sticking to safety guidelines builds trust, especially as customers grow more concerned about what they put on their skin.
Dipropylene glycol goes further than creams and perfumes. Manufacturers add it to cleaning products, where it helps mix ingredients that wouldn’t blend well on their own. Some industrial applications tap into its ability to keep things evenly mixed, making it useful in paints, inks, and hydraulic fluids. Factories and workshops depend on formulas that won’t separate or clog equipment, and this chemical helps meet that need.
In the world of e-cigarettes, it sometimes shows up in the liquid people use to produce vapor. The public health conversation around vaping highlights the importance of making sure all ingredients are used with safety in mind and that users get real information about how products work inside the body.
An ingredient like dipropylene glycol shows how chemistry shapes everyday life. As more people push for clean and sustainable products, manufacturers can continue exploring plant-based or biodegradable ingredients for the same jobs. It takes research and time, but swapping out old standards with greener alternatives matters for customers and the planet. My own experience searching for fragrance-free and hypoallergenic products taught me that transparency and choice go hand in hand.
Quality control, honest labeling, and attention to current science help people make decisions based on facts, not just marketing. If new research ever points to better options for health or the environment, the best companies will adapt. For now, dipropylene glycol keeps creams fresh and fragrant and helps factories run smoothly—reminding us that the science behind the label can be worth a closer look.
Dipropylene glycol often ends up in lotions, creams, perfumes, and even deodorants. I’ve seen it listed on ingredient labels for years, usually buried among terms that sound more chemical than comforting. For a regular person, that can feel intimidating. What exactly is this compound, and does it actually belong on human skin?
Dipropylene glycol is a type of alcohol, related to propylene glycol, which most people have used in one product or another. Its job is pretty straightforward — it helps mix oil and water, stops things from drying out, and ensures products glide on smoothly. In today’s world, almost nobody wants a cream that separates or feels sticky.
So, where’s the catch? Research examining dipropylene glycol’s effects on skin generally paints a very low-risk picture, especially compared with harsher chemicals or known irritants. Dermatologists and toxicologists have long used it without sounding the alarm. According to the Cosmetic Ingredient Review panel, this ingredient doesn’t pose a problem for most people, even when used on sensitive skin.
The US Food and Drug Administration lists it as safe for use in cosmetics, and European regulators agree. It doesn’t usually provoke allergies. In more than a decade of following dermatology updates, it's rare to see dipropylene glycol land on the list of potential troublemakers.
Some people argue that “chemicals” should never touch skin. This doesn’t match what science shows. Everything is a chemical at the molecular level — even water. Problems can emerge with almost anything if used irresponsibly or at massive concentrations well beyond cosmetic standards.
Over the years, I’ve helped friends figure out why a moisturizer caused redness or irritation. Sometimes, dipropylene glycol was present, but almost always with fragrances or other ingredients known for sensitizing skin. I’ve never seen convincing evidence from large, objective studies that points to dipropylene glycol as the culprit in healthy adults. High concentrations in industrial settings do deserve more caution — but cosmetics don’t approach those levels.
No single ingredient works for everyone. People living with eczema or chronic sensitivities sometimes want to skip anything unnecessary. Patch testing helps, and talking with a dermatologist brings peace of mind. When rare reactions do happen, they’re much less common than with preservatives or fragrances. I’ve personally seen more flare-ups from things like sodium lauryl sulfate or parabens.
Products with simple ingredient lists reduce the risk of accidental irritation. Choosing fragrance-free and dye-free formulas can also help. If someone worries about dipropylene glycol, checking product labels and doing a spot test is wise. Hearing from others with similar skin issues in support groups or on forums often gives reassurance, and for most, reactions trace back to different culprits.
Transparency keeps the conversation healthy between companies and customers, especially around ingredients people don’t recognize. There’s room for beauty brands to share results from their own safety tests, or open up direct conversations with dermatologists who know the latest research. If dipropylene glycol ever does become a worry for more than a handful of people, responsible companies will move fast to offer alternatives.
For now, based both on published research and personal experience helping friends and family, most people using products with dipropylene glycol likely won’t face problems. Staying aware, asking good questions, and patch-testing new creams is the best protection anyone can have.
Glycols fill the ingredient lists of perfumes, skincare, and industrial products. Standing in the middle of this maze are dipropylene glycol (DPG) and propylene glycol (PG). Both come from the same chemical family, but their differences do more than just matter on paper—they shape safety, scent, and everyday use.
Propylene glycol is a small, slippery molecule, which means it carries and dissolves ingredients quickly. DPG, on the other hand, is larger and heftier. It doesn’t evaporate as fast and feels less watery. This simple size difference drives much of what sets them apart in the real world.
PG turns up everywhere. You’ll see it on deodorants, in toothpaste, as a carrier in food flavors, and even helping antifreeze do its job in your car. The FDA gives PG the green light as an additive in foods and medicines. I’ve seen people grow wary about the chemical name, but studies say PG is generally safe in the doses we come across. Still, sensitive skin shows its red streaks or itchy spots when PG is around, which talks to us about why alternatives matter.
DPG shows up in more subtle ways. Try reading the back of a bottle of perfume, and there you’ll see DPG as the liquid that cradles all those fancy aroma chemicals. Its slower evaporation keeps fragrances hanging in the air a little longer or helps them linger on the skin. Cosmetic makers lean on DPG for its gentle touch—it’s less likely to stir up irritation, and it feels weightless.
Concerns about skin reactions or swallowing accidents always come up. Most negatives with PG come from large doses or long-term exposure, which almost nobody meets unless working on an industrial scale. DPG’s track record for skin sensitivity looks even cleaner, which explains why many personal care brands switch between both to suit different folks.
Both these glycols end up in sewage after getting rinsed off skin, washed out of pipes, or drifting away from factories. PG breaks down fast in water and air, making it less of an environmental headache. DPG also degrades, although it sticks around a bit longer due to its size. If a company wants a greener image, they’ll favor the one with proven fast breakdown and low toxicity.
Cost shapes a lot in manufacturing. PG tends to run a bit cheaper, given its production scale, but some products demand DPG’s extra weight and lower volatility. My experience in the cosmetics aisle tells me most folks never notice the difference—unless their skin has told them, loud and clear, what it dislikes.
Doctors, chemists, and product designers keep returning to the drawing board looking for gentler, safer, and more sustainable options in these molecules. Choosing between PG and DPG doesn’t just tick a box. It’s a decision that blends chemistry and care, weighing up safety, performance, and the long-term effects—on people and the planet alike.
Anyone who has spent time mixing essential oils or experimenting with homemade scents learns quickly that perfume isn’t just about flashy top notes and fancy bottles. Stability, smoothness, and the way a fragrance lingers tell the real story. Here, dipropylene glycol (DPG) has earned a regular spot on the perfumer’s shelf. I remember walking into a supply shop years ago and asking about it, only to have the shopkeeper slide over a gallon jug and say, “Try it, you’ll see why people won’t work without it.”
DPG works as a carrier and solvent. It takes concentrated fragrance oils, thins them out, and helps the scent disperse more evenly, especially in stickier bases or those finicky musk notes that otherwise grab on and refuse to let go. Since DPG doesn't have much of a smell, there’s nothing to mask or clash with delicate compositions—rose stays rose, sandalwood stays true. Perfume makers keep coming back to it because it solves a problem that water or straight alcohol can’t. It helps distribute a scent without speeding up evaporation or leaving a heavy, oily residue.
Working safely with ingredients matters more than ever. I’ve seen enough allergy scares around new skincare formulas or fragrance launches to know that every ingredient gets scrutiny. Companies don’t gamble with skin contact. The good news: dipropylene glycol scores low on irritancy tests. Regulatory bodies like the Cosmetic Ingredient Review and ECHA keep tabs on it, and the scientific consensus says it doesn’t trigger allergies for most people and breaks down safely after use.
The FDA allows DPG in cosmetic formulations, and reports from IFRA (International Fragrance Association) confirm that it keeps meeting safety standards, so long as suppliers provide high-purity grades—especially the kind labeled “fragrance grade.” Low-grade batches sometimes contain impurities. These get weeded out in reputable supply chains. Seeing trusted brands use DPG in air fresheners, fine fragrances, and even special effects fog machines encourages small makers to follow suit, because why risk it?
The science makes sense, but there’s an art to using DPG. It softens strong oils, stretches the performance of expensive ingredients, and prevents certain notes from browning or changing after a few months on the shelf. Tiny studios trying to balance cost and performance lean on it for keeping batches stable through hot summers and unpredictable shipping. Even bigger fragrance houses mix it with other solvents to fine-tune drying time and silage (how far a scent can travel in the air).
Alternatives exist—fractionated coconut oil, propylene glycol, and plant-based solutions crop up for “clean” branding. Still, none check off as many boxes as DPG when it comes to transparency, low odor, and cost. Perfumers talk about sustainability a lot lately, asking if suppliers use greener chemistry or if any new research reveals better options, but for now, DPG still fills a need.
Retailers and makers can source fragrance-grade DPG and keep purity sheets on file. Labs should rotate stocks, keep containers sealed, and avoid freezing or baking in summer delivery trucks. Clear communication about ingredients reassures customers—simple batch notes or QR codes go a long way. For those looking to “green up” their formulas, supporting research into biodegradable solvents helps push the industry in a better direction. DPG’s place in perfume isn’t about tradition alone—it comes from answering the daily grind of making good scents safer, longer-lasting, and true to their original inspiration.
Walk down any supermarket aisle, grab a scented candle, a stick of deodorant, or that go-to moisturizer, and chances are you’ll spot dipropylene glycol somewhere on the label. This clear liquid appears all over the place—personal care shelves, fragrances, even cleaning products. Working in a soap-making studio years ago, I noticed how often manufacturers rely on it to keep things smelling good, feeling smooth, or dissolving just right. Given its widespread use, concerns pop up: Is dipropylene glycol (DPG) truly safe?
Scientific bodies—including the Cosmetic Ingredient Review Expert Panel—dig into safety data all the time. Reports show DPG doesn’t build up in the body and tends to get flushed out pretty quickly. The U.S. Environmental Protection Agency classifies it as a low-toxicity compound. An average household consumer won’t see obvious health risks when this ingredient pops up in sprays, creams, or styling gels, as long as it’s used as intended.
Years of lab testing have looked for problems like skin irritation or long-term organ effects, and the overwhelming result points to a mild profile. Someone with unusually sensitive skin may notice some redness or itching from products with higher amounts, and breathing in fog machines loaded with DPG during parties or concerts could leave throats scratchy. No mass poisonings. No mystery illnesses traced back to routine use.
Chemical jargon carries baggage; not every familiar-sounding word feels trustworthy. Folks hear “glycol” and link it to antifreeze horror stories or stronger solvents, when DPG’s makeup is quite different. My own neighbors saw the word in room spray and asked if their pets were in danger. The straight answer: Flavor and dosage shape the outcome. Ingesting undiluted DPG—nobody should drink it—will upset stomachs and might cause vomiting, just like scarfing down tubes of toothpaste or eating spoonfuls of raw lotion. For pets or children, accidental big gulps could require a call to a doctor. Most exposure through skin or a scented room poses low risk, according to regulatory documents from Europe and the US.
Big companies hate recalls and lawsuits. They lean on safety thresholds backed by real-world evidence. The European Commission gives DPG the green light for cosmetics and home products under strict limits, focusing on purity testing and batch oversight. The US Food and Drug Administration tolerates it as an indirect food additive (think: packaging or machinery only), not as an ingredient in food you eat directly.
Workplace rules call for good ventilation, gloves, and basic care during industrial handling. No excuses for reckless dumping or mixing it up with foodstuffs at the plant. Fragrance industry standards set safety margins, and watchdogs check for compliance.
If using DPG-laced products leaves skin prickly, switch brands or talk to a dermatologist. Store items away from curious kids and pets, just like any household cleaner. Read ingredient lists—knowledge beats rumor every time. For hobbyists making homemade candles or cosmetics, stick to recommended recipes and don’t improvise. Any cloud of vapor deserves fresh air. Looking for more peace of mind? Public databases like the Environmental Working Group’s Skin Deep offer easy access to safety profiles.
My time in the workshop taught me that “chemical” doesn’t always mean unsafe, and real harm rarely hides in plain sight among regulated everyday goods. Stay curious and keep asking questions about the labels you read.