Diethyl D-(-)-Tartrate has been around for well over a century. Its roots go back to the golden age of organic chemistry in the 19th century, a period when European chemists raced to uncover and classify natural compounds. Louis Pasteur, in his early work, famously separated tartaric acid crystals by hand, laying the groundwork for the modern understanding of chirality. This attention to right- and left-handed molecules directly opened the door for diethyl tartrate and its use in asymmetric synthesis. Industrial manufacture took off in the 20th century, especially as pharmaceuticals demanded higher-purity building blocks and how the fine chemicals industry needed scalable, reliable sources for chiral compounds. Lab technicians and industry experts collaborated to standardize the preparation and purity requirements to keep up with ever-stricter safety and application standards in areas like medicine, agricultural chemicals, and flavors.
Diethyl D-(-)-Tartrate, a colorless liquid ester, stands out in organic synthesis thanks to its two stereocenters and the reliable optical rotation it offers. Chemists routinely reach for this compound when developing new catalysts, especially in the process of creating enantiopure substances—critical in medicine, where the stereochemistry of a molecule can mean the difference between a blockbuster drug and a dangerous compound. The substance maintains a consistent reputation because of its solid regulatory track record, time-tested methods for purification, and years of research into its numerous derivatives. From my own experience with asymmetric synthesis, Diethyl D-(-)-Tartrate shortens the trial-and-error typically faced when isolating one enantiomer from a racemic mixture; its efficiency saves both time and money on the lab bench.
Diethyl D-(-)-Tartrate presents as a clear, oily liquid with a mild, pleasant smell, useful if you handle chemicals daily and want to avoid strong odors. It melts at around -12°C and boils close to 289°C, which offers a reasonable safety window during distillation or solvent removal. Its density hovers around 1.2 g/cm³, and it's only slightly soluble in water but freely mixes with many organic solvents. The optical rotation, a distinguishing mark of this substance, stays close to -7.5° to -8.5° (neat), letting analysts check consistency and batch quality with simple polarimetry. Stability-wise, it resists hydrolysis under standard lab conditions but will slowly break down in the presence of strong acids or bases—something I learned after forgetting a flask on the bench overnight. Diethyl D-(-)-Tartrate’s functional groups—two ester moieties—react predictably, making downstream modifications reliable and efficient in synthetic routes.
Precise labeling ensures safety and compliance, especially where product purity determines the outcome of a reaction. Manufacturers typically deliver the compound at a purity above 98%, and reputable sources provide data sheets showing water content, optical rotation, and any impurity traces. Labels carry critical handling information: keep it dry, protect from sunlight, store at room temperature in a tightly-sealed bottle, and avoid sources of ignition. Supply chains have improved traceability, with lot numbers and manufacturing dates ensuring accountability all the way to the lab fridge. Meeting industry test requirements—for residual solvents, heavy metals, and enantiomeric excess—means the same level of care you see in pharmaceutical production extends to this chemical. Technical specifications reflect both regulatory pressure and growing end-user expectation for clear, transparent information.
During preparation, the process typically follows esterification of naturally derived tartaric acid using ethanol in the presence of sulfuric acid. The method favors cost and scalability, employing a straightforward condensation reaction under controlled heating. Any large-scale industrial synthesis employs efficient separation steps—often distillation and liquid-liquid extraction—to remove water and excess alcohol, improve yield, and drive the reaction to completion. Purification uses distillation under reduced pressure to avoid thermal decomposition. Purification steps sometimes need column chromatography to deliver the high optical purity that pharmaceuticals and advanced material applications expect. Getting hands-on with this process early in my career, those small details—such as drip rate of acid or even the room temperature drift—could shift yields significantly, so careful attention pays off.
The ester groups in Diethyl D-(-)-Tartrate serve as the basis for transformation into a range of useful intermediates. Common reactions include transesterification—swapping out the ethyl groups for bulkier alcohols—or saponification to free the tartaric acid, which then re-enters other synthetic routes. Under mild conditions, the compound undergoes selective reductions or acts as a ligand in metal-catalyzed asymmetric reactions. Sharpless asymmetric epoxidation employs it as a chiral modifier to create enantiopure epoxides from allylic alcohols, a cornerstone reaction in modern pharmaceutical chemistry. From my perspective, the low reactivity of the ester bonds under neutral conditions keeps accidental side reactions in check—a welcome relief for those chasing ever-complex molecules with tight tolerances on purity and isomer ratio.
Other labels for Diethyl D-(-)-Tartrate pop up throughout the literature and chemical catalogs. You might find it under “(2R,3R)-Diethyl 2,3-dihydroxybutanedioate,” “Diethyl L-(−)-tartrate,” or “(+)-Wine acid diethyl ester.” Language shifts depending on regulatory domain and local naming standards, but knowledgeable chemists recognize the core D/L designation and the importance of the stereochemistry. This clarity on nomenclature matters most when ordering for research projects where a labeling mix-up could mean days of lost effort, spoiled batches, or findings that fail a peer review for lack of reproducibility.
With safety at the forefront, users approach Diethyl D-(-)-Tartrate with basic PPE—lab coat, goggles, nitrile gloves. Skin contact causes mild irritation, and spills clean up with ethanol or isopropanol. Inhalation risks stay low under normal ventilation, but concentrated vapors irritate mucous membranes. Emergency measures include eye-wash stations and fire extinguishers rated for alcohols. Storage regulations focus on flammability—secure bottles, no exposure to open flames, routine inspections for leaks or label wear. Waste disposal follows state and federal guidelines for organics but rarely poses a high environmental risk if handled correctly. Over the years, adherence to these ordinary standards keeps workplace incidents rare, and routine safety meetings reinforce correct handling practices, especially for new technicians or students.
Pharmaceutical research and production take full advantage of Diethyl D-(-)-Tartrate’s chiral purity. It finds regular use in synthesizing cardiovascular drugs, beta-blockers, and specialty antibiotics, where a small impurity can trigger side effects or render drugs ineffective. In agricultural chemistry, crop protection agents grow safer due to precise stereochemistry, avoiding off-target activity. The compound enhances flavors and fragrances, imparting subtle notes or helping stabilize volatile esters in complex blends. Research institutions see steady demand for the substance in developing new catalysts, where one batch may underpin years of experiments. Having spent time in a custom synthesis lab, the demand for enantiomerically pure tartrates keeps growing as regulations tighten and discovery pipelines require sharper selectivity.
Innovation in asymmetric catalysis leans heavily on Diethyl D-(-)-Tartrate as a supporting ligand or precursor. Recent advances in organocatalysis, green synthesis, and biocatalytic resolution often cite the compound as a key building block. Ongoing R&D work explores new ways to improve process yields, minimize waste, and boost the sustainability of precursor feedstocks by sourcing tartaric acid from fermentation byproducts instead of chemical synthesis. I've watched universities shift their organic chemistry curricula to include hands-on modules with tartrate esters, furthering the skills pipeline for future scientists. Industrial partners respond with investment in more streamlined, lower-emission production facilities that lower costs and environmental impact.
Toxicologists assess Diethyl D-(-)-Tartrate as having relatively low acute toxicity when handled with standard precautions. High doses affect the intestinal tract, with nausea and diarrhea noted in animal studies, but human exposure remains low due to the compound’s limited volatility and infrequent use outside controlled lab settings. Chronic studies in rodents do not reveal clear carcinogenic or mutagenic effects. Environmental fate studies show ready biodegradation in soil and water, which reduces ecological persistence. Regulatory bodies, including the European Chemicals Agency and US EPA, review toxicity data to set workplace exposure limits, though current assessments regard the compound as safe for typical industrial and research use cases. Safety reviews continue as product use expands, but hands-on familiarity reassures researchers who rely on established handling protocols.
Looking forward, Diethyl D-(-)-Tartrate stands as a cornerstone for new asymmetric synthesis, especially as personalized medicine and green chemistry come to the forefront. The compound’s continued utility hinges on scalable, low-impact production routes and wider recognition of its role in complex molecule construction. Researchers investigating renewable sourcing anticipate integrating microbial fermentation or enzyme-based transformations to further cut resource consumption. As automated and digital chemical synthesis platforms become mainstream, reliable chiral standards such as Diethyl D-(-)-Tartrate underpin data quality and reproducibility. Speculation in the sector points to applications in materials chemistry, such as chiral liquid crystals and advanced electronics, pushing the molecule beyond traditional pharmaceutical confines. The expansion of global markets for chiral intermediates, coupled with regulatory clarity and robust safety data, secures a vital role for Diethyl D-(-)-Tartrate in the years ahead.
Folks who spend much time in chemistry labs know the real value of a chemical that solves everyday challenges. Diethyl D-(-)-tartrate fills this role better than most. It’s a clear, oily liquid that packs more punch than its simple makeup would suggest. Chemists often reach for it when making certain drugs or separating left-handed from right-handed molecules, a process that too often gets overlooked outside the lab.
Sometimes, small differences make a huge impact. Take pharmaceuticals. Many medicines have molecules that can twist one way or the other—picture your hands, similar but not the same. Only one ‘hand’ usually brings about the effect you want. Diethyl D-(-)-tartrate helps chemists sort these shapes out. If you’re taking a medication, there’s a good chance chemists used this ingredient somewhere behind the scenes to help shape the final product.
Ask anyone who’s worked in a synthetic chemistry lab, and you’ll hear how this material greases the wheels for important reactions. One of the most common uses shows up in the Sharpless epoxidation. That’s a mouthful, but in plain language, it’s a reaction that lets scientists build complex molecules with remarkable control. This is huge for drug discovery, where even small errors can lead to big setbacks. Diethyl D-(-)-tartrate helps tip the odds toward success by favoring the right structure, one that leads to safer and more effective drugs.
There’s another side to the story. Industries beyond pharmaceuticals lean on this chemical to create flavors, fragrances, and specialty materials. Creating complex scents or precise flavoring agents for food requires separating the right-shaped molecules. If you enjoy that unmistakable aroma in a favorite perfume or the taste in a familiar candy, there’s a chance diethyl D-(-)-tartrate played a quiet but essential role somewhere down the line.
Some folks hear the word ‘chemical’ and immediately worry. It’s true—no chemical should be handled carelessly. Diethyl D-(-)-tartrate requires gloves, good ventilation, and an understanding of proper disposal. Safety data from the manufacturers back this up, and reputable labs make this a top priority. These rules aren’t an obstacle—they’re what keep people safe while letting industries keep making progress. The real lesson here is that respect and routine make all the difference between safe use and trouble down the road.
The world wants safer chemicals and better ways to make them. That pursuit will never stop. Green chemistry encourages scientists to create safer routes and fewer byproducts. Some new research looks at alternative catalysts that reduce waste and make the process cleaner. Progress in this space could bring greener solutions to the table without sacrificing the effectiveness that diethyl D-(-)-tartrate offers.
In everyday life, most people never give much thought to the behind-the-scenes work that delivers medicine or a favorite food flavor. Yet, diethyl D-(-)-tartrate shapes these outcomes more than most folks realize. Good science, steady respect for safety, and an eye on the future help ensure these good things keep coming, with even better methods just around the corner.
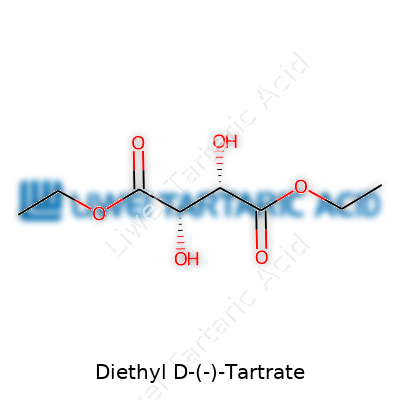
Many folks outside a chemistry classroom might glance at a name like Diethyl D-(-)-Tartrate and feel their eyes start to glaze over. This compound has a big part to play in labs, across industries, and even in university lectures. The chemical formula—C8H14O6—might look like a random mix of letters and numbers, but it carries meaning and helps chemists work smarter.
No set of symbols in a chemical formula gets chosen by accident. In this case, you’ve got eight carbon atoms, fourteen hydrogens, and six oxygens stacked in a certain structure. Walk through any research lab: chemists use the formula to predict reactions, purity, and the compound’s fit in a new synthesis.
Diethyl D-(-)-Tartrate is an ester, meaning it comes from tartaric acid, where two ethanol molecules are hooked onto the core. Those extra ethyl groups tweak how it behaves, making it useful in synthesis and separations—especially where chirality matters. If you’ve spent time working with chiral reagents, you know how critical stereochemistry can be. Getting the wrong isomer can wreck a synthesis or undercut a pharmaceutical trial.
Handedness in molecules isn’t just a trivia fact for chemistry tests. The D-(-) means this compound bends polarized light in a certain direction and sits on one side of the tartaric acid family tree. Enantiomers—molecules that look like mirror images—can have wildly different effects in the body, or in industrial processes. Medicinal chemists, for example, pay close attention to get the right version, since the wrong one could be inactive, or sometimes even dangerous.
When companies in pharmaceuticals or fine chemicals hire scientists, they look for people who understand not just the names and formulas, but why the right isomer saves time and money. It’s not just about making the reaction work. It’s about avoiding waste, improving yield, and sometimes making sure a medicine actually treats what it’s supposed to.
Production of Diethyl D-(-)-Tartrate comes from tartaric acid, itself derived from naturally occurring sources like grapes. Wine lovers might not care much about esters, but chemists find practical uses—this compound steps up as a chiral building block or as a resolution agent to separate other molecules that look almost identical. In my graduate days, seeing how a slight twist in structure could drive selectivity shocked me. For synthesis, that precision translates to better products, less byproduct, and cleaner processes.
The safety angle should never be ignored. Even though its origins are natural, it’s a lab chemical needing respect. Breathing dust or vapors or letting any organic ester collect in high concentration spells trouble without proper ventilation and gloves. Safety data sheets back that up.
Appreciating a formula like C8H14O6 stretches beyond rote memorization. Chemists, educators, and anyone in industry get more out of their work by seeing how structure ties to function. The complexities of isomers, the impacts on downstream reactions, and even the choices made for greener chemistry connect back to knowing and understanding these building blocks. The formula of Diethyl D-(-)-Tartrate symbolizes that deep link between theory and practical, everyday science.
There’s a common thread in every chemistry lab, whether in a sprawling pharmaceutical factory or a tiny startup’s garage. Every flask or bottle tells its own story, but the wrong storage often erases it before the ink is dry. That’s true for Diethyl D-(-)-Tartrate. Many folks glaze over storage advice—keep it cool, keep it dry—like it’s background noise. After seeing a few costly lab mishaps, it’s clear why nobody should ignore those two words.
This chemical’s pretty standard in organic synthesis, especially for chiral resolutions. Most folks assume a bottle sealed with a plastic or glass stopper is good enough. Water vapor thinks otherwise. Moisture doesn’t crash through the front door; it seeps in, day by day. Diethyl D-(-)-Tartrate loves to soak it up, slowly changing what’s inside that bottle. Before you know it, you’re troubleshooting reactions that worked just fine last month but flop today.
For folks running reactions that demand strict stereochemistry, even a bit of water or hydrolysis products can turn a well-planned project upside down. You stop trusting your chemicals, and confidence in your work takes a hit just when you need it most.
Some might shrug and say, “It’s not that volatile.” The truth is, air can do more than just sit there. Oxygen gives Diethyl D-(-)-Tartrate trouble over time. It can cause slow oxidation, especially if the lab gets hot or sunlight hits the bottles through a window. Suddenly, you deal with off odors and degraded product.
I once watched a colleague toss half a batch because the faintest yellow tinge crept in after a summer on a sunny windowsill. That day taught clear lessons: dark, sealed cabinets beat fancy storage rooms unless you shell out for specialty fridges.
The best labs make the extra effort to store this ester in bottles with tight-fitting caps, tucked into desiccators or on well-labeled dry shelves. Toss in a few silica gel packets and you give yourself breathing room. Keep it away from heat sources—not next to radiators, not on top of chemical hoods that double as storage spots.
The purists park their tartrate in cold rooms, often at 2–8°C. This slows hydrolysis and minimizes the slow crawl of time and air. But most of us work with what we have. Keeping the bottle sealed, out of direct sunlight and away from sinks or chemical baths, gets the job done for months. If you pop the container open every day, smaller bottles help cut down exposure.
After a few ruined reactions and more than one quality control frustration, you remember the basics aren’t busywork. One mistake with storage means questions about every subsequent result. Proper storage of Diethyl D-(-)-Tartrate isn’t a mere box-check exercise. It builds trust in the rest of your chemistry and keeps the mistakes out of your notebook and off your budget sheet.
Simple habits—label dates, use dry storage, avoid sunlight, respect the fridge if you have it—pay off more than any magic bullet additive. In a field where every variable counts, proper care of this ester lets your ideas, not your storage, drive your next success.
Diethyl D-(-)-tartrate often appears in laboratories and chemical manufacturing, mainly as a chiral building block for making other substances. Chemists reach for it because its molecular shape helps craft drugs and specialty chemicals with specific handedness. It isn’t something people run into at home or at work unless the job involves chemistry or pharmaceuticals.
Exposure determines whether any chemical becomes dangerous. The Material Safety Data Sheet (MSDS) gives us a starting point. Diethyl D-(-)-tartrate isn’t acutely toxic in small amounts, but that never tells the whole story. Swallowing, breathing in, or letting it soak into your skin can irritate tissues—just like many esters or solvents do. Splashing it in your eyes triggers pain and watering. I once spilled a related ester on my glove and felt my skin tingle for hours, reminding me that lab chemicals deserve respect even when labeled “low hazard.”
Some data suggest that tartrate esters can upset the gastrointestinal system if swallowed, leading to nausea or cramps. Inhaling concentrated vapors sometimes causes dizziness or headache, especially inside a poorly ventilated lab. Chronic high-dose contact has not been widely studied in people, but animal data show no strong evidence of lasting harm such as cancer or birth defects. That’s a relief, but lack of proof doesn’t guarantee total safety.
Packing, storing or transporting this chemical usually requires standard protective measures: nitrile gloves, a lab coat, and good eye protection. The smell is faint and fruity, so you might not notice spills right away. If it touches your skin, soap and water help remove any residue, but leaving it too long may cause redness.
Working with chemicals like Diethyl D-(-)-tartrate gives a clear lesson: even seemingly low-risk compounds can cause harm if handled carelessly. Studies have shown lab workers underestimate risk for “routine” chemicals compared to strong acids or bases, but accidents often happen when people relax their guard. My own experience with minor burns and spills, fortunately mild, left me cautious for life.
The environmental footprint of diethyl tartrate is modest, yet it doesn’t break down instantly if poured down a drain. Routine disposal into sewer systems puts unnecessary strain on water treatment facilities. In concentrated form, it could harm aquatic organisms, especially if major spills reach the environment. Environmental protocols in labs require collecting and recycling or incinerating leftover material.
Simple habits lower risk more than fancy equipment. I always double-check labeling and caps before and after pouring. Labs train staff to use fume hoods, even for "safe" esters, since vapor exposure over time causes headaches in enclosed rooms. Skin contact happens less often by keeping gloves clean and switching them out if they get splashed.
If working outside a lab—rare, but possible for manufacturers—folks should stick to similar habits: avoid skin and eye contact, don’t eat or drink nearby, clean up spills immediately.
Regulation and safety training make a difference. Chemistry instructors and supervisors do well guiding less experienced colleagues away from complacency. Companies also provide more robust Material Safety Data Sheets than they once did, listing symptoms, first-aid tips, and correct disposal methods. Alternatives to diethyl tartrate exist for some processes, though price or chemical performance sometimes keeps it in use.
Taking even seemingly “mild” chemicals seriously protects not only our health but also that of our communities and environment. In thousands of labs worldwide, this simple precaution matters far more than any official label of “hazardous” or “toxic.”
Diethyl D-(-)-Tartrate plays a key role in both research and industry, especially for chemists working on asymmetric synthesis. The compound’s quality determines if a reaction can meet its target or goes sideways. Purity is more than just a data point on a certificate — it’s the backbone of reliable results, from pharmaceuticals to high-value fragrances. Labs and manufacturers know that a pinch of contamination can derail a whole batch, causing both financial and reputational headaches. This explains why purity standards aren’t just for show; they decide who trusts a batch and who rejects it.
The most respectable producers offer Diethyl D-(-)-Tartrate with a purity of at least 99%. You search for this figure in product literature or certificates of analysis, knowing that every decimal matters. This specification means the product contains 99 grams of the desired substance in every 100 grams — no surprise contaminants, no mysterious leftovers. High-performance liquid chromatography (HPLC) remains the gold standard for confirming these numbers because it picks out even the faintest trace of unwanted substances.
Moisture content often gets its own line on the certificate. Most labs set a maximum, usually at or below 0.5%. Even a splash of water where it doesn’t belong can mess with reactions, so this matters as much as the main purity number. The optical rotation, too, shows up often, as it speaks to the correct tartrate isomer. Chemists use a polarimeter to spot-check those values and confirm everything lines up with D-(-)- configuration, instead of its mirror image.
It’s easy to think this kind of strict oversight only matters for the big guys. I’ve seen small producers and hobbyists run into trouble after trying to cut corners with off-spec material. In pharmaceuticals, nobody accepts any risk of impurity, which could trigger failed batches, unexpected side effects, or worse. The same goes for the food sector, where trust in the safety and source of ingredients shapes not just product quality, but also long-term business.
The implications go beyond safety. Productivity and cost control both hinge on using high-purity material. Impure tartrate can slow down a process, force extra purification steps, or trigger recalls. One story rings in my mind: A contract manufacturer threw away thousands of liters of solvent after discovering minor contamination that originated from the tartrate supply. Each step of neglect in the supply chain multiplies risk for everyone afterward.
No lab or factory leaves purity to chance. Procurement teams always ask for certificates of analysis and spot-check them before signing a contract. Larger outfits might go further, running their own HPLC or NMR checks on samples. I’ve witnessed some buyers visiting suppliers to review manufacturing protocols, auditing everything from storage rooms to QA logs.
Transparency about sourcing helps everyone. Serious manufacturers map out the origin of every ingredient and keep full documentation available. This builds the trust chain back to the original producer, making it clear who stands behind the quality. In recent years, regulatory bodies have stepped up, demanding full traceability, especially after high-profile contamination cases shone a light on what can go wrong when corners are cut.
At the end of the day, pure Diethyl D-(-)-Tartrate allows companies big and small to keep promises to customers — and to themselves. Anything less opens the door to risk, waste, and frustration, so the industry-wide push for stringent purity specs makes practical and ethical sense.