Dichloropropanol has a long history in the chemical industry. Starting in the mid-20th century, researchers sought chlorinated solvents and intermediates for developing plastics and other synthetic materials. Scientific papers in the 1950s described new ways to prepare intermediates for epoxide and epoxy resin production, and dichloropropanol often popped up in those studies. As the demand for polyether materials and herbicides grew, manufacturers found dichloropropanol to be a useful building block. Over time, the processes used to manufacture and isolate dichloropropanol became more reliable and efficient thanks to plant optimization and new purification technologies. The development paralleled the broader move towards synthetic materials and chemicals used in agriculture, construction, and manufacturing, reflecting society’s drive for productivity and innovation in daily life.
Dichloropropanol comes in several forms, with 1,3-dichloro-2-propanol being the most widely produced. Chemists and plant managers use it as an intermediate, mainly where downstream processes need both chlorine atoms and an accessible alcohol group. You can find it as a colorless to pale yellow liquid, offering moderate solubility in water and good compatibility with non-polar solvents. Facilities package and transport it in drums or bulk tanks, labeling the containers clearly to underscore its hazardous nature. Across North America, Europe, and East Asia, chemical firms include dichloropropanol in product lines supplying the plastics, resins, and agricultural chemicals markets.
On the laboratory bench, dichloropropanol stands out with a boiling point that lands between 160–190°C, depending on the isomer. The molecular weight, just over 112 g/mol, means it pours easily and volatilizes more slowly than lighter solvents. That slow evaporative rate suits controlled reactions, where operators want to avoid dangerous vapors. Its density, just above water, means spills can be managed with common containment methods. Despite feeling a bit oily, it clings less easily to surfaces than heavier chlorinated solvents. Its reactivity comes mainly from the two chlorine atoms, which break free in nucleophilic substitution reactions, and from the alcohol group, which couples readily with acids and bases in both laboratory and industrial setups.
Producers ship dichloropropanol with Certificates of Analysis, reporting purity, water content, and any residual byproducts from the synthesis. Quality standards from organizations like ASTM and the European Chemicals Agency keep levels of impurities—like residual dichloropropene or heavier chlorinated byproducts—well below thresholds known to cause issues downstream. Storage drums carry lots of safety labeling: chemical formula, hazard and precautionary symbols, risk phrases, contact information for poison control, and batch traceability. Some larger customers test incoming batches for trace chlorinated residues to head off product recalls or process shutdowns.
Manufacturing dichloropropanol starts with propylene, a three-carbon basic petrochemical feedstock. Reacting propylene with chlorine under controlled light or heat creates propylene dichloride, which then undergoes controlled hydrolysis. Chemists typically use carefully dosed water, acid catalysts, and temperature cycling to target the conversion to dichloropropanol. Getting high yields requires tight attention to temperature and pH since the process can easily produce polychlorinated byproducts if left unchecked. Modern reactors use in-line sensors and feedback controls to keep the process stable, and any off-spec batches get cycled back for reprocessing to keep material losses low.
In pilot-scale and production chemistry, dichloropropanol’s value comes from its reactive positions. Nucleophilic substitution—where a chloride swaps with a hydroxyl or alkoxy group—creates epichlorohydrin, a key intermediate for making epoxy resins and glues. Sometimes plants convert dichloropropanol with alkali, stripping away one chlorine atom to close the carbon chain into an epoxide ring. Other applications harness the alcohol’s reactivity, grafting acids or other alcohols onto the molecule’s core to build up more complex structures for advanced resins or specialty chemicals. The molecule’s chlorinated sites also mean it can act as a precursor for introducing more functional groups in multi-step synthesis.
People in the trade know dichloropropanol goes by a few other names. Common alternatives include “DCP,” “1,3-dichloro-2-propanol,” and, in some markets, “glycerol dichloride.” There’s sometimes confusion between 1,2- and 1,3-isomers, since each acts a bit differently in reactive processes. Trade literature sometimes shortens the name to “dichloropropanol” without the number, which can cause headaches for purchasing and shipping teams. Label clarity not only reduces confusion but keeps supply chains tight and safer, making it vital to check product codes before accepting any new consignment.
Handling dichloropropanol means taking health and safety seriously. Direct skin, eye, or respiratory exposure can lead to irritation, and, at higher concentrations, cause severe systemic toxicity. Many workplaces require gloves, goggles, and full-face shields for workers dealing with open containers or pipes. Facilities ensure good ventilation in storage and process areas to keep airborne concentrations below recommended limits set by agencies like OSHA and NIOSH. Written emergency plans detail the right steps for spills: ventilation, containments, neutralization solutions, and personal protective equipment at the ready. Some plants run regular evacuation drills and rapid decontamination sessions, reinforcing the idea that nobody should treat hazardous chemicals casually.
In everyday manufacturing, dichloropropanol plays a role most people never see. It’s a backbone chemical for making epichlorohydrin, which feeds directly into epoxy resin production. Those epoxies form the coatings, adhesives, and composites found in electronics, cars, wind turbines, and construction projects. Agrochemical manufacturers turn to dichloropropanol as a raw material for certain herbicides and pesticides, adding value to crops and helping meet global food demands. In laboratories, researchers use it for exploring new organic synthesis routes or testing novel reaction pathways. Its ability to introduce chlorine atoms and create reactive intermediates keeps it in steady demand among specialty chemical firms looking to meet evolving industrial and agricultural needs.
Research teams and chemical engineers keep looking for less polluting, more energy-efficient ways to produce dichloropropanol. Labs across Europe and Asia have tested new catalysts that cut unwanted byproducts and lower reaction temperatures. Some groups experiment with bio-based feedstocks, reducing demand for fossil-derived propylene while still delivering the needed molecular structure. There’s steady interest in safer derivatives and process modifications, both to meet tighter regulatory controls and give downstream customers products with improved performance or environmental profiles. Academic groups keep reporting novel reactions involving dichloropropanol—often looking for greener chemistry or more selective routes to complex, high-purity final goods for electronics and biomedical engineering.
Scientific studies on dichloropropanol’s toxicity have revealed some real concerns. Repeated exposure can harm the liver, kidneys, and central nervous system. Animal data suggest it can act as a potential carcinogen at high doses or long durations, driving regional authorities to set strict occupational exposure limits. Toxicologists keep a close eye on metabolic and breakdown products, since chlorinated solvents sometimes linger in tissues or groundwater, increasing the risks to both workers and the environment. Calls for more in-depth human health studies have grown, especially for at-risk populations involved in production, transport, or disposal. Treatment protocols and industrial hygiene rules continue to adapt as new health data emerges from animal and cell culture tests around the world.
Moving forward, demand for dichloropropanol will likely trend with big shifts in plastics, coatings, and global infrastructure construction. Regulatory pressure pushes manufacturers to clean up production lines, both through investments in emission controls and by designing safer, more selective chemical processes. Technology advances like digital plant monitoring and artificial intelligence open doors to finer control over quality, yield, and safety across chemical plants. Green chemistry approaches—to cut hazardous byproducts or use renewable feedstocks—might shake up traditional supply chains over the coming decade. As societies demand more sustainable products, producers face a balancing act: keeping pace with changing laws, protecting workers and the environment, and meeting the practical needs of industries that rely on high-performance resins, glues, and coatings. The next generation of chemists, plant engineers, and regulators will have plenty to work on if they want to keep this useful but challenging chemical in check.
Dichloropropanol stands out as a chemical compound with more than one form, but most of the talk revolves around 1,3-dichloropropanol (1,3-DCP). You won't find it on grocery shelves or in medicine cabinets—it's really a tool used in industrial processes. Manufacturers rely on it for making epichlorohydrin, which then gets turned into epoxy resins. Those resins end up everywhere: coatings, adhesives, sealants, electronics, even water pipes. In my time working with construction professionals, there’s hardly a conversation about strong, reliable adhesives that doesn’t circle back to products made from epoxy resins.
The biggest ticket item for dichloropropanol is its role as an intermediate step in making epichlorohydrin. Epoxy resins formed from epichlorohydrin keep wind turbine blades strong and lightweight, give smart devices their protective shells, and help keep pipes sealed against leaks. Sometimes, it ends up as a byproduct in other jobs involving chlorine or glycerol. The chemical world is messy that way—rarely does anything exist without leftovers or side reactions.
A handful of companies use dichloropropanol in the synthesis of other chemicals, especially in making surfactants (cleaning products), pesticides, and pharmaceuticals. My uncle, a chemical engineer, told stories about plant managers arguing over tiny impurities like 1,3-DCP in their product streams—even at a few parts per million—because those traces may end up in finished products or waste. That tells you how seriously regulated substances like this get treated.
Long experience in environmental testing gets you used to looking out for certain names—1,3-DCP pops up in risk lists because it carries toxicity concerns. Some forms can be harmful if breathed in, swallowed, or absorbed through skin. Governments watch closely for it, especially where food safety is involved, because trace amounts have turned up in some soy sauces during quality checks. Some contamination can sneak through when food processing uses chemicals or recycled water. The European Food Safety Authority and other agencies set strict limits for residues in foods because animal studies showed it could increase cancer risk.
Laws in Japan, Europe, the US, and several other regions consider dichloropropanol a priority contaminant. So food companies test sauces, drinks, and even snacks to keep levels low. Workers in chemical factories wear protective gear because repeated exposure has been linked to liver and kidney issues and possible carcinogenic effects. My own time in labs reminded me: take chemical safety seriously, or somebody ends up in the ER.
Cleaner manufacturing often means going back to basics—find ways to make chemicals without creating unwanted dichloropropanol on the side. Some companies switched out older chlorine-heavy processes for newer, more controlled reactions using less harmful inputs. Regular monitoring, updated filtration at food plants, and constant air and water sampling cut down on accidental leaks. I’ve seen big brands overhaul raw material sourcing just to reduce the risk of any hazardous chemicals wandering into their finished goods.
Education stays key. Plant workers, food producers, and even grocery buyers ought to know the risks and demand transparency. Tighter rules help, but only if tested often enough to catch problems before they grow. From the ground up, it’s people—whether in white coats or not—asking questions, chasing cleaner processes, and keeping eyes open that make the biggest difference.
Most folks don’t stop to consider how chemicals sneak into daily life. Yet, names like dichloropropanol, especially 1,3-dichloropropanol, often grab headlines in food safety debates. This compound comes up because it’s a byproduct in processed foods, especially where fats get treated at high heat or with certain additives. Industrial workers might also run into it during production of epichlorohydrin, which ends up in resins and plastics. What sticks out here: both direct and indirect paths exist for people to run into this chemical.
Researchers working on chemical safety label dichloropropanol as worth extra attention. I remember reading health studies from organizations like the World Health Organization and the International Agency for Research on Cancer. Their research shows 1,3-dichloropropanol causes tumors in lab animals. Not a minor detail, since that raised questions about whether it can trigger cancer in humans, too.
Long-term exposure—a big worry for workers and people who often eat foods made industrially—links to higher cancer risk. Even short bursts, such as a spill at work, may lead to headaches, dizziness, and skin irritation. Nobody wants their salad oil or soy sauce laced with something that can hurt the body at even low exposure over years.
Europe set strict residue limits in food, and Asian nations rushed to do the same after several food scares. Despite rules, food testing sometimes still reveals low levels of this compound. It makes sense for health experts to push for transparency—if people know how much shows up in everyday food or workplaces, they gain power to weigh risks, especially for folks with kids or chronic health problems.
Whether in an office job or working in a warehouse, trust builds from honesty about risks. In a younger job, I had friends in local factories who never got told about what drifted in the air or what landed on their skin. Simple updates and non-technical education could have gone a long way for them. The same applies to chemicals like dichloropropanol. Clear warnings and labeling, regular workplace air testing, and routine health checks for workers matter most. People avoid trouble if they catch exposure early, not after years of low-level contact.
On the food front, more robust checks and public databases on residue testing give families peace of mind. Food companies benefit, too, since trust helps long-term business. In my kitchen, seeing labels that clearly say what’s inside scores big points. Nothing’s worse than feeling tricked about health.
Safer methods exist for processing oils and fats that cut down formation of risky byproducts. Tech innovation, plus some old-fashioned transparency, reduce exposure on two fronts. Research shows that switching production processes in food factories slashes how much dichloropropanol forms. For heavy industry, using better ventilation and ensuring staff wear proper protection works as a basic line of defense.
In short, folks deserve to know what they might eat or breathe during a shift at work or home. Anyone involved—whether scientist, food producer, or consumer—shares responsibility. Taking these steps means safer living for everyone, not just those reading scientific journals or policy reports.
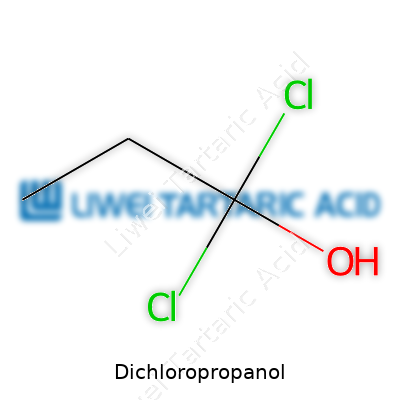
Dichloropropanol catches the spotlight in chemistry labs thanks to its role as a building block for other chemicals. Its structure is all about the carbon backbone and the way chlorine atoms latch on. The basic chemical formula for dichloropropanol is C3H6Cl2O. You’ll often see two main isomers: 1,2-dichloro-2-propanol and 1,3-dichloro-2-propanol. Isomers make a difference — their properties change slightly based on where the chlorine and hydroxyl groups sit on the chain.
That formula, simple as it looks, links to some big responsibilities. Dichloropropanol doesn’t show up by accident on a commercial site. It’s usually a step on the way to producing more recognizable products. For example, the chemical industry often relies on dichloropropanol to make epichlorohydrin, a key input for epoxy resins. Every time I repair something with epoxies — whether it’s a leaky pipe or a cracked gadget case — I feel grateful for the chemistry involved upstream.
Still, not many people realize that substances like dichloropropanol slip into the picture as byproducts, especially in processes that involve chlorine and propylene or glycerol. Those steps generate residues that demand careful management, both for environmental and occupational health.
Digging into safety data brings up some concerns. Chlorinated alcohols like dichloropropanol don’t just vanish. Studies show that prolonged exposure might cause liver toxicity, and animal studies raise questions about carcinogenicity, although direct links remain under investigation. Strict exposure limits and air-handling systems pop up in facilities that handle these chemicals.
Experience teaches that ignoring even a seldom-seen compound can backfire. I’ve walked through industrial sites where waste stream management makes all the difference. Runoff loaded with chlorinated compounds, even in tiny amounts, ends up causing regulatory headaches — and, worse, health risks for communities living nearby.
People sometimes brush off chemical formulas as dry details, but each number and letter carries a ripple effect through the supply chain. Tracing dichloropropanol from its formula to its use in manufacturing shows that chemical literacy matters beyond academic circles. As someone who’s spent years around manufacturing, I’ve watched best practices around containment and environmental safety keep accidents at bay.
Cutting down unwanted byproduct formation and investing in advanced waste treatment can soften the impact of chlorinated substances. Several companies use catalytic processes designed for higher selectivity, which helps reduce dichloropropanol waste. Others partner with researchers on greener chemistry, like swapping to less hazardous raw materials or recycling spent solvents.
Diving into the realities behind C3H6Cl2O opens up plenty of connections to health, the environment, and responsible industry. Understanding the formula isn’t just memorization — it’s part of navigating a world where chemistry quietly shapes everyday products.
Dichloropropanol isn’t a word many of us toss around every day, but for people who work with industrial chemicals or manage storage spaces, it carries real weight. The stuff brings hazards—breathing it in, letting it leak, contact with skin. Stories have surfaced where poor storage led to eye irritation, skin burns, even fires. These moments should remind us of the importance of laying safety groundwork before something goes wrong.
One lesson I learned early from a veteran warehouse manager: never trust a rusty drum. Strong, tight-sealing containers make all the difference. Steel or high-density polyethylene works well, since both materials resist corrosion and keep leaks in check. Instead of makeshift lids or reused barrels, always use containers specifically labeled for hazardous chemicals—no room for shortcuts there. Clear, tough labels help workers spot the right drum fast, and avoid painful mix-ups.
Dichloropropanol gives off fumes that you don’t want floating around. A hot, stuffy storeroom makes evaporation and pressure build-up way more likely. A dry, cool room—ideally below 25°C—slows it all down and keeps vapors in check. Good ventilation plays a part, too; one well-placed exhaust fan can mean the difference between safe air and a strong chemical stink. Wet spaces also invite corrosion, so keep it bone-dry if you can.
The chemical reacts with strong oxidizers, some acids, and even water. For this reason, arrange storage away from things like bleaches, peroxides, or anything that builds heat easily. No flammable liquids nearby. Sprinklers aren’t the best fire-fighting option because dichloropropanol and water don’t mix well—dry powder or foam extinguishers work much better on accidental fires. Keep this in mind before packing a room full of chemicals together in a rush.
No storage space stays safe by accident. Routine checks catch leaks before they reach the floor. Watch for discoloration, dents, or distended barrels. Take those warning signs seriously and swap out damaged containers right away. If a spill occurs, staff should have practiced the response already—spill kits, protective gloves, goggles, and lots of absorbent material ready to go. More than once, I’ve seen chaos spread when no one knew where the cleanup gear sat.
Even the best container can’t prevent mistakes if folks don’t know the guidelines. Short, regular training sessions back up awareness. People remember better when they see eye-catching signs and real-life examples, not just rulebooks. Don’t leave new hires to figure it out alone—walk them through storage rules, explain label meanings, and rehearse emergency steps.
Making safe storage second nature may take time, especially if fast-paced work seems to push safety aside. But a bit of planning keeps accidents in check. Rely on solid procedures, quality containers, and a well-trained crew, and you’ll keep dangerous surprises at bay. Safe storage isn’t glamorous, but it keeps everyone healthy—and in the end, everyone gets to clock out in one piece.
Dichloropropanol often shows up in chemical labs, industrial settings, or wastewater treatment, prized for its use in synthesizing other chemicals. Most people won’t cross paths with it, but for those of us working with chemicals, it pays to know how quickly mishandling turns nasty. Even brief skin contact can cause irritation or burns. Breathing in the vapors causes headaches, dizziness, or worse. There's talk of cancer concerns with long-term exposure. Anyone working near this compound should stay alert to these dangers.
Some workers think gloves or goggles slow things down, but experience shows skipping protection comes back to bite you. Nitrile gloves hold up better than latex when handling organic solvents, and splash-proof goggles become a must once you crack open a drum or bottle. In crowded shop spaces, even bystanders run a risk if vapors escape. Lab coats and closed shoes may look old-school, but I’ve seen enough ruined jeans and skin rashes to know those safeguards pay off.
No fancy lab required – even temporary setups can boost safety with the right airflow. A working fume hood pulls away harsh vapors before you ever catch a whiff. An open window just doesn’t cut it since heavier gases can hang near the floor. It’s far easier to set up local exhaust fans or vented hoods than manage a health crisis after someone gets exposed.
Spills don’t stay put. They soak into porous benches, make floors slippery, and send vapors everywhere in seconds. Best move: grab a chemical spill kit fitted with absorbent pads and neutralizers, not just a paper towel. My old workplace kept a clear protocol taped to the wall. Anyone who saw a spill had orders to evacuate the area, alert a supervisor, and gear up with respirator masks. After clean-up, proper disposal never got skipped, since drains and trash cans became off-limits for anything toxic.
A messy shelf brings as much trouble as a careless spill. Cool, well-ventilated racks far from direct sunlight make a safe home for chemicals. I’ve learned to check labels and double-check inventory dates, since rusted caps or cracked bottles leak vapors before you can see them. Segregating acids, bases, solvents, and flammables cuts down the odds of a reaction that could end in disaster. Any container with missing info lands straight in the hazardous waste bin.
Older workers pass along habits to newer staff, but formal safety training beats word-of-mouth every time. I put trust in well-designed sessions that walk through chemical handling, label reading, equipment checks, and emergency steps. Regular reviews and drills keep those lessons fresh. Safety officers at my previous job posted new guidelines by every entrance, and it helped everyone stay sharp.
Accidents do happen. Quick eye washes, showers, and emergency numbers on speed dial limit the fallout. Our shop kept antidotes close and required spill-response drills twice a year. Speed counts: rinsing eyes or skin fast makes the difference between a minor irritation and a hospital trip. Workers who know where to run and what to do make the whole team safer.