Back in the day, chemists were mixing up all sorts of esters trying to carve out better results for food, pharma, and specialty chemicals. D-Tartaric acid, dug up from natural sources like grapes, always grabbed attention for its chiral purity in shaping other molecules. Esterifying tartaric acid meant chemists could tune its solubility or reactivity for different jobs, and the diiso-propyl ester started cropping up as glassware filled with bubbling, sweet-smelling batches. Demand for optically pure compounds in asymmetric synthesis, especially in European labs during the mid-20th century, made this ester a staple. Later, as industry leaned heavier on precise chiral technologies in agrochemicals and drug development, the processes sharpened to favor higher yields, easier workups, and better safety controls.
D-Tartaric Acid Diiso-Propyl Ester lands under the class of organic esters made from pairing D-tartaric acid with isopropyl alcohol. Often sold as a colorless to pale yellow liquid, it carries a faintly fruity smell. What sets this particular version apart: its specific chirality, which allows chemists to lock in the 'right-handed' qualities they want for reactions. That’s especially true in pharmaceutical resolution work or prepping for catalysts. The molecule isn’t just a boring solvent or filler. It’s an integral building block in some of the most demanding chiral applications out there—think custom catalysts or fine chemicals.
Pour a sample and you'll find it's a low-viscosity liquid at room temperature, with a boiling point somewhere in the range of 280°C. It dissolves neatly in most organic solvents but steers clear of water—hydrolysis tends to break the ester bond. The density shows up around 1.09 g/cm³, and the refractive index tips close to 1.420. The chirality isn’t just a technical footnote either; you’ll see it show up in optical rotation, with strong positive readings confirming its D-form. The primary allure for technical users lies in that combination of purity, stability under storage, and the ease with which it takes part in further reactions.
Suppliers stake their reputation on keeping enantiomeric excess above 98% to guarantee nobody ends up with racemic waste in a synthesis. Most packaging shows batch numbers, production dates, purity percentage (by GC or HPLC), and a clear indication this is the D-form, not the meso or L-isomer. Shelf-life runs a couple of years with proper sealing. Wholesale batches come in amber glass or HDPE drums, always labeled for hazard class due to its moderate flammability and potential irritating properties. The flashpoint informs storage standards, while trace impurity levels (especially residual solvent and water) matter for pharmaceutical buyers.
Producers go for esterification of D-tartaric acid using excess isopropanol under acid catalysis. Sulfuric acid remains the favorite catalyst, often run under reflux to drive off water and push the equilibrium right where it needs to go. Removal of isopropyl hydrogen sulfate byproduct and excess alcohol calls for careful distillation under reduced pressure—nobody wants to lose optical activity by overheating. Some labs switch to Dean-Stark traps or run azeotropic distillation to squeeze out that last bit of water. Afterward, companies typically run the crude ester through silica to grab any leftover acid or colored impurities, yielding a sharp, focused product ready to box and ship.
One thing stands out in practice: this ester shows remarkable stability under mild base and neutral conditions but breaks apart with strong acids or bases, freeing up tartaric acid and isopropanol. Chemists often tap into this for stepwise protection and deprotection in building more complicated molecules, especially in the synthesis of chiral ligands or specialty reagents. It can even act as a resolving agent, helping to separate racemic amine bases during salt formation. These properties prove handy for both large-scale pharmaceutical plants looking to make enantiomerically pure APIs and research outfits designing custom catalysts.
This compound doesn’t go by a single name in the market. Some users call it D-(+)-Tartaric acid diisopropyl ester. Trade catalogs toss around 'Isopropyl D-tartrate' or less formally 'diisopropyl tartrate.' CAS Numbers and EC registry numbers get attached for tracking and regulatory paperwork, which means that chemical safety data sheets have to match up with product names carried on customs manifests and technical ordering documents. These aliases matter when cross-referencing studies, as even scientific papers sometimes mix up D-isomers with meso or racemic listings.
Handling this ester doesn’t come without strict rules. Gloves and goggles remain must-haves in any professional lab, since spills can cause skin and eye irritation, even though it’s not classified under the worst toxic hazard scales. The ester’s low volatility makes vapor inhalation less of a concern, but larger volumes mean good fume hoods and proper ventilation. In the event of a spill, teams lean on absorbent pads instead of washing it down the drain, protecting local waterways. Storage keeps to cool, dry spaces—UV and excess heat speed up breakdown and could bump up peroxide risks. Regulatory compliance trails every step, with adherence to REACH, OSHA, or China’s SAWS standards determining not only worker safety but insurance rates too. Emergency kits stock eyewash and spill-neutralizing granules to minimize panic if an accident happens.
Most users see this compound as indispensable in asymmetric synthesis, especially for resolving racemic amines, alcohols, and acids. It’s a mainstay in research labs in both academia and industry, supporting projects in organic synthesis, catalysis design, and even polymer engineering. Pharmaceutical teams rely on it for prepping chiral intermediates, while agricultural chemists find it useful for molecule tweaks that make pesticides and herbicides safer and more effective. In my own time working with chiral columns and optical purity analyses, I’ve run across this ester in more than one purification process, never failing to appreciate how much simpler it made the work compared to older, less selective options. Its flexibility has even opened doors in perfumery and flavors for preparing rare esters not found directly in nature.
Teams never stop hunting for better catalysts or ways to run selectivity up and waste down. A lot of attention turns to green chemistry—using less hazardous reagents or recyclable catalysts to clean up the traditional synthesis method. Some research outfits focus on enzyme-catalyzed esterifications, where milder conditions make for safer workspaces and simpler waste handling, though scale-up still trails behind. Analytical advances let labs check purity and enantiomeric excess in real-time, trimming downtime. You can follow the research trail in academic journals, where the rush to find more efficient chiral auxiliaries and resolution agents still features D-tartaric acid esters front and center.
Animal studies suggest this ester doesn’t cause acute toxicity at low exposure, though repeat, high-dose contact can cause kidney impact similar to other esters. Regulators in the US and EU tend to group it under general irritants, more worried about accidental exposure than chronic poisoning. Emergency posters in facilities stress washing off quickly in the event of spills—especially for those with sensitive skin or pre-existing respiratory concerns. Long-term occupational studies remain limited, but regular handling protocols include risk assessments, medical surveillance, and employee training to address unseen concerns. I’ve never seen a major industrial accident tied directly to this compound, though workers who ignore PPE sometimes report mild headaches or rashes.
Looking forward, commercial use of D-Tartaric Acid Diiso-Propyl Ester grows with the demand for specialty fine chemicals, especially as synthetic and pharmaceutical chemists push deeper into precision medicine and more rigid regulatory setups. Green chemistry tweaks—like solvent-less reactions or catalysts based on recyclable materials—aim to cut costs and push for safer workplaces. Applications in drug development, agrochemicals, and materials science keep expanding as new uses for custom chiral molecules pop up. I expect research into biocatalytic routes will keep gaining traction, not only to limit waste but to let smaller labs and startups jump into production without heavy capital investment. Monitoring environmental fate and occupational safety stands as the last big hurdle before the compound sees even wider mainstream adoption, moving from niche to necessity in the toolkit of synthetic chemists everywhere.
D-Tartaric Acid Diiso-Propyl Ester isn’t something most folks have sitting around the house, but go behind the scenes in a pharmaceutical research lab, and you’ll find it playing a starring part. Chemists often prize this specialty ester for helping separate out molecules that look nearly identical but behave very differently. In drug work, one form of a molecule can fight disease, while its mirror image might be useless or even do harm. This ester lets scientists prep the right side of a chemical puzzle, trimming chances for complications later on.
Pharma companies use this ester when they want to control how a molecule twists. Having a handle on this step means medicines work better and cause fewer headaches during trials. Ask anyone in drug development about time spent fixing issues with molecular shapes—they’ll tell you how much easier life gets using tools like this one. It’s about saving effort, saving money, and staying safe.
Specialists in organic chemistry go to D-Tartaric Acid Diiso-Propyl Ester when they want to make custom chemicals. Whether someone’s putting together building blocks for flavorings, fragrances, or agrochemicals, they face the same problem: getting only one version of a molecule. This ester lets them take short-cuts without falling into tricky dead-ends. The selective power of D-Tartaric Acid Diiso-Propyl Ester makes life easier for anyone who spends hours at the bench, turning raw materials into advanced or fine chemicals.
Its predictable behavior removes plenty of guesswork. In the hands of someone who knows what they’re doing, this means higher yields and purer results. Less wasted starting material. Fewer failed batches. These advantages matter both for scientists chasing prizes and for businesses working to lower their expenses.
The food supplement world doesn’t always get the spotlight in stories about specialty chemicals, but D-Tartaric Acid Diiso-Propyl Ester still pops up. Makers of vitamins or nutrition products often want ingredients that hit strict purity standards, especially for supplements shipped around the world. Getting the right chiral form isn’t just a regulatory step—it’s about knowing exactly what ends up in the bottle.
Consumers deserve pills and powders with dependable results, and it’s tools like this ester that help guarantee each batch checks out. It’s not glamorous, but it keeps the trust flowing between companies and their buyers. Firms that get this right earn a solid reputation and cut down on expensive recalls.
Producing specialty esters can create waste, and no one working in chemistry ignores that problem now. Many labs search for cleaner ways to handle these reactions. Some groups now push to recycle reagents or use less-toxic solvents during production of D-Tartaric Acid Diiso-Propyl Ester. This work continues as regulations tighten and demand rises among buyers who care about their supply chain’s footprint.
If chemists can keep refining these methods or find bio-based options, D-Tartaric Acid Diiso-Propyl Ester might hold its place as a workhorse chemical while becoming more sustainable. Strong industry standards and smart partnerships with suppliers can help. In the end, that means safer labs, better products, and a smaller impact on the environment.
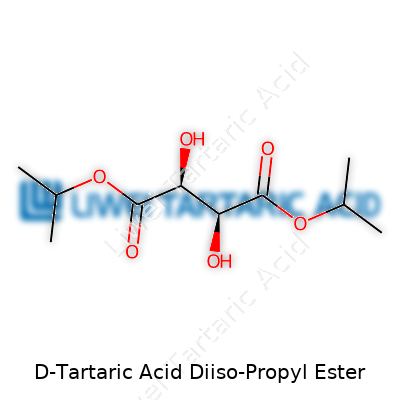
D-Tartaric Acid Diiso-Propyl Ester brings a certain intrigue to chemistry benches, especially when the discussion drifts to stereochemistry. With the chemical formula C12H22O6, this compound starts out as D-tartaric acid, a naturally chiral molecule found in grapes and other fruits. Story goes, tartaric acid played a part in the birth of stereochemistry thanks to Louis Pasteur’s legendary work with its crystals. Turn it into a diester using isopropanol, and you arrive at the diiso-propyl ester. The backbone remains the four-carbon tartaric acid, each carboxyl group now linked to an isopropyl group through an ester bond. Imagine looking at a carbon chain lined with two hydroxyl groups on the second and third carbons and two isopropyl esterified carboxyls at the ends.
The configuration matters — this is D-tartaric acid at play, so you’re dealing with a molecule where the chiral centers both have the same orientation. To draw the structure, you’d see:
On paper, the structural formula often reads: (CH3)2CHOOC–CH(OH)–CH(OH)–COOCH(CH3)2. In three dimensions, the D-form gives the compound a twist that sets it apart from the L-form and the meso analogue. The two isopropyl groups, jutting out at each end, impact both solubility and volatility. That’s more than just academic — those properties influence solvent choices and how you handle the compound in actual applications.
Plenty of folks can rattle off a chemical formula, but boots-on-the-ground chemistry demands thinking about how those atoms are arranged. D-tartaric acid’s configuration decides everything from optical rotation to how it interacts in enantioselective syntheses. Esters like this show up in making chiral ligands and resolving agents. In pharma, a single stereoisomer can spell the difference between helping a patient and causing trouble — the wrong stereoisomer of a compound, after all, can mean no activity or even toxicity. D-tartaric acid derivatives have a proven track record in finding, isolating, and synthesizing pure chiral compounds. Years ago, I worked in a lab using tartaric acid esters as resolving agents for chiral amines. Results always confirmed the need for clarity over configuration.
Safety data for tartaric acid esters calls for a level head. Esters tend to give off vapors; in confined spaces, irritation grows into a real risk. Disposable gloves and eye protection stand as basic habits. Tartaric acid itself is low hazard, but esters, especially volatile ones, can sneak into the air. Storage in airtight bottles and using them in fume hoods just makes sense. Handling derivatives with care preserves both your results and your health.
Sourcing or producing D-tartaric acid diiso-propyl ester asks for more than a line drawing. Reliable suppliers carry thorough paperwork on chirality, purity, and trace contaminants. Checking for optical activity and verifying with chromatographic analysis makes a difference when each enantiomer counts. In my experience, labs that neglect this step spend more time troubleshooting than making headway.
Esters like D-tartaric acid diiso-propyl ester play a role in green chemistry as well. The core tartaric acid can be sourced from winemaking waste, putting a positive spin on what would otherwise be landfill. Switching to greener solvents and cutting down on hazardous byproducts matters for today’s chemistry labs, too. Plus, new catalytic and enzymatic methods for esterification reduce the energy load of making these molecules. The tools for more sustainable synthesis are already in reach.
D-Tartaric Acid Diiso-Propyl Ester is not something most people bump into at the supermarket. Out in the real world—labs and manufacturing floors—this stuff plays a role in making specialty chemicals and pharmaceuticals. I’ve spent enough time around chemical storage areas to catch problems people might miss. Chemicals like this don’t just “sit” quietly if ignored. Sooner or later, someone pays for a shortcut.
Plastic and glass work, though not every plastic can handle glycol esters. Polyethylene and glass containers typically earn trust. Old coffee cans and repurposed water jugs just bring headaches, not savings. Reliable containers save people from leaks, spills, and ruined batches. I remember hearing about a technician pouring a clear liquid from a repurposed bottle with no label. Turned out, he wasn’t even sure what he was handling. A quick fix is never worth a burnt hand or a ruined pair of shoes.
Storage away from sunlight and heat sources keeps things stable. Stuffing bottles onto a window ledge invites trouble. Heat means vapor pressure climbs, stressing caps and seals. In one crowded storeroom, a forgotten flask of solvent puffed up so badly it split the lid—chemical fumes aren’t just unpleasant, they come with real health risks. Sticking to a cool, dry spot—ideally below 25°C—avoids a lot of drama, and a locked chemical cabinet stops curious hands from picking up something dangerous.
Clear labeling on every bottle stops confusion. Handwritten tape wears off, so printed labels work better. Include the name, date received, and “hazard” information, such as flammability or toxicity. Sharply written labels make it obvious—no one forgets which container carries D-Tartaric Acid Diiso-Propyl Ester.
Face shields and chemically resistant gloves beat single-use nitrile gloves every time for this job. Eye protection turns a splash from a disaster into a minor clean-up headache. Years ago, I watched a coworker try to wipe something from his eye after a splash; that hospital visit convinced me to never skip goggles. Spill kits with absorbents and neutralizers belong close to the storage area. Quick cleanup means less risk and less lost time.
Good airflow makes a difference. Small spills or slow leaks get pulled away from noses and lungs with real ventilation—not just working next to an open window. Walk into a poorly ventilated supply closet and you taste yesterday’s chemistry. Fume hoods and local exhaust fans mean headaches and coughing don’t follow you home.
Training beats posters every time. People who understand how D-Tartaric Acid Diiso-Propyl Ester behaves react faster if something leaks or spills. Written protocols posted on the wall—step-by-step for transfer, cleanup, and waste disposal—create a safer workplace. Inspections once a month and updating old labels keep the system honest. I’ve seen a single “reminder” meeting stop a year’s worth of preventable accidents.
Safe storage and smart handling prevent injuries and protect everyone’s hard work. Nobody enjoys a panic when a hazardous chemical gets loose. These commonsense habits don’t take much time, but they deliver peace of mind every day on the job. Respecting the hazards builds trust, keeps the work moving, and lets everyone go home in one piece.
Once you step into a lab or set foot in a production plant, you realize quickly that the details in a material’s purity can shift the entire outcome. D-Tartaric Acid Diiso-Propyl Ester, with its technical name and a role in pharmaceuticals and syntheses, proves this point. Lab-scale chemists, quality control teams, and plant managers rarely choose chemicals in a one-size-fits-all way. They ask questions: What does this batch contain? Will it mess up a reaction or taint a final product? That sort of awareness leads to different grades and qualities landing on the market shelf.
Factories producing high-performance coatings or pharmaceutical intermediates don’t want traces of anything that doesn’t belong. Even micro-contamination can ruin a batch that costs thousands—sometimes millions. Chemical suppliers learned this through both industry demand and hard lessons. Some offer D-Tartaric Acid Diiso-Propyl Ester at ≥98% purity for pharmaceutical work. Others focus on technical or industrial-grade, which may contain more side products, but also shaves down costs. There’s no mystery about why these distinctions exist—money spent on extra purification goes somewhere, and some users just don’t need that level of purity.
A chemist working on sensitive enantioselective syntheses can’t gamble with leftovers from production. For them, purity isn’t hype. It’s about reliability, so a small amount of impurities could push a reaction off the rails or leave them cleaning up a nasty chromatography mess. On the other hand, a company using the ester in large-scale plastics might not care about a percent or two of byproducts that end up burnt off or transformed during processing. The key question always comes back to: What’s the end use, and what happens if there’s a contaminant?
At one point in my early lab work, our batch yield dropped without warning. Tracing it, we found out our supplier switched to a “technical grade” of an ester. Though the difference sat below 2%, the reaction selectivity tanked. The error didn’t just waste time—it hit our research budget, tested patience, and set the whole team on edge. That burn sticks with you. So does the lesson that price tags can hide far bigger costs if corners get cut on purity.
Some brands post full certificates of analysis, but buyers shouldn’t just accept a sheet at face value. It pays to push for batch-specific analytics, thorough impurity profiles, and a dialogue with suppliers who understand the stakes. There’s a push in the industry toward green chemistry—solvents, reagents, and esters with clearer traceability and less waste. Regulators increasingly call for tighter scrutiny, especially if the ester sits in a drug intermediate supply chain.
End users need to define what’s acceptable and make it part of their purchasing agreements. Some labs set up incoming quality checks, others invest in backup suppliers. In every case, the risk sits with those who skip the finer details. The conversation on grades and purity in specialty chemicals never really goes away. It just adapts with the challenges, technology, and, above all, hard-won experience.
Chemicals like D-Tartaric Acid Diiso-Propyl Ester don’t show up in everyday conversations, but they end up in a lot of labs and factories. Too often, it’s easy to lose sight of basic safety issues, especially when a substance’s profile isn’t widely publicized. Personal safety goes hand-in-hand with knowledge. If you’re handling this ester or working around it, you should start by reading a material safety data sheet (MSDS). These reports highlight skin irritation, eye contact risks, and inhalation hazards you can’t afford to overlook.
My years in laboratory research taught me that even pure-looking liquids can deliver a punch if splashed onto skin or into the eyes. For D-Tartaric Acid Diiso-Propyl Ester, exposure often triggers mild to moderate irritation. Eyes redden and sting, and skin contact sometimes leaves a rash or surface burn. Regular use of gloves and goggles never felt optional—too many stories in academic circles prove the point.
Breathing in vapors deserves equal attention. This compound doesn’t give off a strong odor, so by the time you notice exposure, discomfort or headache might already set in. Fume hoods take the guesswork out of the picture. There’s no shortcut for using proper ventilation, especially in rooms where you can’t open a window.
Chronic exposure brings its own concerns. Repeated skin contact could create allergies over time, and while there’s limited public data on whether this ester poses carcinogenic risks, the best policy is old-fashioned caution. I once trained under a professor who insisted on rotating staff when handling chemicals with unknown chronic effects. This practice protected us from repeated exposure and probably kept his safety record spotless.
On the environmental front, esters like this one have a knack for slipping into water systems, either through improper disposal or accidental spills. They don’t always break down quickly. Local fish and water organisms become unintended casualties if factories let wastewater standards slide. Water treatment plants can filter out the big offenders, but trace amounts still add up—making chemical stewardship more than a buzzword.
Toxicology data from trusted sources, such as Sigma-Aldrich and the National Center for Biotechnology Information (NCBI), tally up the most common side effects: irritation to skin and mucous membranes, and a low risk of serious systemic poisoning. Animal studies haven’t flagged acute toxicity at standard concentrations, but missing information doesn’t equal safety. As with many specialized esters, data on reproductive harm or neurological effects lags behind, especially for people facing years of on-the-job exposure.
Solutions start with basics: gloves, goggles, and lab coats. Well-maintained exhaust systems pull fumes and vapor away from workers. Safety showers and eyewash stations stand by for emergencies. Training sets expectations before new hires touch a single drop. Chemical storage shapes safety culture—label everything, segregate incompatible chemicals, and use secondary containment to halt leaks.
From my point of view, plain communication saves the most injuries. Let everyone in the workplace know what to look out for, and spur management to fund regular safety audits. Supporting a culture where speaking up about unsafe practices brings praise instead of blame keeps the hidden dangers in check, making workplaces safer for everyone.