Caprylhydroxamic Acid grew from the persistent problem of finding gentle yet effective preservation in cosmetic formulations. Older preservatives like parabens and formaldehyde releasers triggered widespread allergies and raised consumer concern, which left developers searching for something milder. In the late twentieth century, chemists started looking into amino acid derivatives, hoping to uncover a safer solution. Caprylhydroxamic Acid emerged from this wave of research—more specifically, the need for preservation in water-based personal care products without strong allergens or harsh antimicrobials. As the "green beauty" trend caught on, Caprylhydroxamic Acid found eager backers in natural and clean product developers. It’s now a staple in the alternative preservative toolbox, showing that targeted chemistry and consumer demand can change the ingredients we rely on in everyday routines.
Most people won’t recognize Caprylhydroxamic Acid on a label, yet it has become a secret weapon for product formulators. It's not a stand-alone preservative but operates in tandem with others, especially in paraben-free or natural systems. Many brands—both indie and multinational—lean on it to resist fungal contamination in creams, serums, and makeup. This ingredient pairs with glycols, like Caprylyl Glycol, to hold back the growth of yeast and mold. Its solubility suits modern cosmetic bases, which are high in water and low in alcohol. You can find it disclosed in everything from luxury moisturizers to everyday shampoos, tucked away among emulsifiers and humectants. That rise in use shows not just an industry trend but a real shift in how cosmetics are formulated for better shelf life with lower health risk.
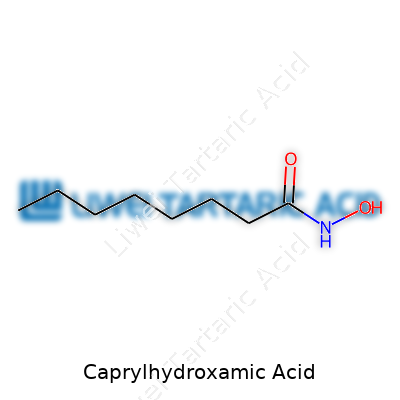
Caprylhydroxamic Acid appears as a solid, usually white or off-white, with a faint, characteristic odor. The molecule comes from caprylic acid, an eight-carbon fatty acid, and a hydroxamic acid group. In chemistry terms, it’s neither too greasy nor too acidic, which lets it blend into most emulsions with ease. It dissolves in hot water and alcohol, although mixing can require gentle heating for full dispersion. Its melting point hovers between 80–85°C, and it shows moderate stability under storage conditions found in warehouses or home bathrooms. It remains active within a pH range of 4 to 7, which covers most leave-on and rinse-off cosmetic products. Its moderate size and polarity help it move through both water and oil phases, so formulators rarely struggle to keep it suspended or distributed.
On global supply lists, Caprylhydroxamic Acid often carries a minimum purity over 98%. Impurities seldom top 1%, as quality standards in cosmetics demand consistent results for both safety and performance. The INCI—International Nomenclature of Cosmetic Ingredients—lists it as “Caprylhydroxamic Acid,” and regulatory bodies in the US, EU, and Asia recognize it as a preservative. Typical usage runs between 0.05% to 0.2% by weight, with labels reflecting either “preservative,” “fungistatic agent,” or the INCI name alone. Some suppliers offer it in wettable powder or pre-dispersed in glycols to assist with production scale-up. Each safety data sheet details storage—cool and dry, out of light—and handling, with clear notes on protective gear for industrial mixing. Proper labeling isn’t just red tape; it’s a way to trace quality back to the batch, which proves key when recalls or audits hit.
The preparation involves reacting caprylic acid (octanoic acid) with hydroxylamine hydrochloride under controlled temperature and basic conditions, classically using sodium methylate or similar bases. The resultant Caprylhydroxamic Acid is then separated, washed, and purified through crystallization. Each step must keep water content low and control pH, or the desired product may degrade into byproducts. On a commercial scale, manufacturers scale up this batch or continuous process, using stainless steel reactors and vacuum filtration. Raw material quality and reagent ratios matter since minor contaminants can foul both the process and the final product. Anything ending up on a consumer’s skin starts with careful, almost daily attention inside plant labs, where chemists chase both the highest yield and the purest crystals.
The hydroxamic acid group in Caprylhydroxamic Acid confers its main function: strong chelation of metallic ions. In typical cosmetic systems, it scavenges iron and copper, preventing those elements from fueling microbial growth or causing product discoloration. The molecule’s hydrophobic tail lets it lodge in oil droplets, further disrupting fungal cell walls, especially those from yeasts and molds. Chemists tinker with esterification or co-formulations to adjust the molecule’s solubility and compatibility. Recent patents show blends with glycols or other hydroxamic acids, aiming for broader antimicrobial spectra without reliance on formaldehyde donors. Direct chemical modification isn’t a big focus—Caprylhydroxamic Acid, as designed, tends to have the right blend of oil and water properties for its purpose.
You might spot Caprylhydroxamic Acid labeled as CHDA, Octanohydroxamic Acid, or by its CAS number: 7377-03-9. Certain trade names reflect proprietary mixtures—companies market blends with glycols or other preservatives as “alternative preservation systems.” Yet the INCI name always pops up in ingredient listings as required by law. In my experience, brand formulators often mention it just as “CHA” in formulation paperwork. The variety of synonyms mostly helps suppliers and regulatory agencies trace supply sources and batch records, and less so for average shoppers.
Manufacturers operate under the close watch of safety guidelines. The CIR (Cosmetic Ingredient Review) panel, along with EU SCCS reports, find the preservative safe for leave-on and rinse-off products within concentration limits. They require proof that final formulations don’t allow for skin penetration or cause eye irritation. Facility operators keep careful records on temperatures, weights, and storage times, since slight process slips can lead to out-of-spec material. Personal protective equipment, local exhaust ventilation, and regular environmental monitoring all come standard in chemical plants making Caprylhydroxamic Acid. Each product shipment comes with up-to-date safety data sheets, so warehouse and logistics teams can respond if there’s a spill or exposure incident. For the consumer, the record looks reassuring—patch test data and post-market surveillance find only rare irritancy cases, usually in sensitive skin or high-use scenarios.
Applications stretch across skin care, hair care, sun creams, wipes, and cosmetic wash-offs. Caprylhydroxamic Acid largely handles the fungi that threaten to spoil water-rich formulas, which proves vital as brands move away from parabens and formaldehyde-based systems. It pairs up with Glyceryl Caprylate or Caprylyl Glycol in “natural preservative” blends that keep products stable and safe, even under warm bathroom or shipping conditions. You’ll find it in facial moisturizers, BB and CC creams, makeup removers, liquid soaps, and even baby lotions, since mildness rules in those categories. The steady expansion of clean beauty options depends on ingredients like Caprylhydroxamic Acid that promise both performance and a low chance of skin problems.
Research keeps moving forward. In academic journals and industrial white papers, teams investigate microbial resistance and broader efficacy claims. Instead of waiting for problems, formulation scientists stress-test Caprylhydroxamic Acid under high humidity, repeated bacterial exposure, or UV light, documenting every result. Some developers work on encapsulation or slow-release systems, hoping to lengthen shelf life even further. Environmental impact studies keep tabs on breakdown products in wastewater, while market researchers look into consumer attitudes toward newer preservatives. Notably, global regulatory harmonization plays a part—brands exporting to new markets must meet different standards, sometimes tweaking formulas with new partners or suppliers. Product developers lean into ingredient synergy, searching for preservation blends that solve the “clean” demand without sacrificing shelf stability.
Toxicologists put Caprylhydroxamic Acid through the wringer under controlled trials. Common tests include rabbit and human repeat insult patch studies, dermal and ocular irritation screens, and full spectrum genotoxicity assays. At the levels used in personal care goods, results so far show low systemic toxicity and virtually no bioaccumulation. Reports flag mild irritation at higher doses or when combined with certain fragrance allergens, but standard formulas don’t trigger these responses in most people. The persistent concern—shared by any new preservative—is the risk when consumers use multiple products daily over years, exposing the same small skin area repeatedly. Studies keep tabs on this possibility, reviewing cumulative exposure and looking for subtle anaphylactic or delayed onset effects. So far, Caprylhydroxamic Acid looks as safe as, or safer than, the compounds it replaced, but vigilance doesn’t let up just because early numbers look good.
Looking ahead, Caprylhydroxamic Acid stands to get even more traction. The global shift into green and sustainable beauty isn’t slowing, and regulatory pressure to cut out legacy preservatives only widens its reach. Brands keep searching for powerful, broad-spectrum systems that play well with naturals, limit irritation, and hold up under global shipping conditions. Some university labs experiment with fermentation-derived Caprylhydroxamic Acid, aiming for improved sustainability and lower environmental load. Data sharing between companies and watchdog groups deepens the knowledge pool—what worked five years back grows old fast when product returns or emerging science signals a tweak is due. Makers see room for new application forms, such as powder and solid formats, which could suit waterless cosmetic launches or extend shelf life in hot markets. If Caprylhydroxamic Acid keeps performing and safety results continue to reassure, chances are it’ll keep its role as a cornerstone in modern personal care preservation strategies.
Walk down the personal care aisle these days, and labels hit you with names that feel more at home in a chemistry lab than your bathroom. Caprylhydroxamic acid stands out among them. It’s a preservative booster, popping up in serums, lotions, shampoos, cleansers, and even some makeup. Folks in the skincare industry lean on it because of its special knack for keeping mold, bacteria, and yeast out of water-based formulas. There’s a technical explanation for how it works, but the takeaway is simple—products stay fresh longer and you avoid rubbing something potentially unsafe on your skin.
Years ago, most products relied on parabens, and before that, formaldehyde donors weren’t uncommon. Both raised alarm bells about possible safety risks, from hormone disruption to outright irritation. So, the hunt began for safer, gentler alternatives. Caprylhydroxamic acid checks this box. It springs from coconut oil, and though it’s a lab-made version, it’s friendly to both the skin and the planet. I remember testing sensitive-skin lotions loaded with harsh preservatives and feeling my own skin crawl by day three. After switching to formulas preserved with caprylhydroxamic acid, those flare-ups all but disappeared.
Plenty of dermatologists and chemists stand by the safety track record of caprylhydroxamic acid. Regulatory groups in the United States, Europe, and other regions have looked closely at its use in cosmetics. So far, no big red flags have popped up related to common usage levels. But every ingredient has its limits. Get too much of anything, and someone’s bound to react. In my work with beauty brands, we’ve always run patch tests and kept the concentration under one percent, which matches guidelines seen in peer-reviewed studies. For everyday folks, the chance of irritation remains low, especially compared to older preservative blends.
Small changes make a real dent in the way products perform and how long they last on store shelves. Caprylhydroxamic acid stands up against a broad range of microbes, so companies don’t need to throw in a whole cocktail of preservatives. This simplicity matters more than it sounds. Fewer ingredients mean fewer chances for someone to have a reaction. Plus, formulas get more flexible—crucial for brands looking to promise “clean” or “gentle” products, both of which shoppers increasingly want. After all, none of us want to deal with spoiled cream or break out because our moisturizer started growing something invisible.
No single compound solves every shelf life or safety issue in beauty. Brands still have to check the source of their caprylhydroxamic acid, keep up with new data, and blend it carefully with other ingredients. Customers play a part by tossing out old lotions, not dipping fingers in jars, and paying attention to how their skin reacts to new stuff. More research helps, especially as products and formulas evolve. Caprylhydroxamic acid fills a real need, letting people try new textures, softer products, and water-rich creams without sacrificing peace of mind. Products stick around longer, and people worry less about what’s lurking in their favorite bottle. That’s a win for everyone, from casual shoppers to folks who spend weekends mixing up beauty formulas in their kitchens.
Caprylhydroxamic acid has a job that matters in modern skincare. This small molecule acts as a preservative, stopping the growth of mold and bacteria in watery formulas. Beauty brands have put it to work as an alternative to parabens, given the ongoing interest in cleaner ingredients. Safety concerns from parabens and formaldehyde-releasing agents led to a real search for something less controversial. As labs started swapping older preservatives with caprylhydroxamic acid, everyone wanted to know if the trade-off gave us something better—or brought hidden problems.
Caprylhydroxamic acid keeps face washes and creams fresh, but skin stays at the center of the conversation. Studies published in peer-reviewed journals—including work seen in the International Journal of Toxicology—report that this ingredient ranks low on irritation and allergic reactions compared with harsher options. For someone who reacts strongly to traditional preservatives, this sounds like good news. Yet, anyone with very sensitive skin knows even gentle ingredients can be unpredictable.
Real-life stories suggest that skin doesn’t always play by the rules set in the lab. People with conditions like eczema or rosacea report flare-ups from products that use newer preservatives—caprylhydroxamic acid among them. The European Scientific Committee on Consumer Safety looked at all the latest evidence and put strict limits on how much could be used in a finished product, usually less than 1 percent. These restrictions aim to lower the risk, but they don’t wipe out all side effects. Once irritation or stinging shows up, even at low concentrations, it’s best to stop using the product.
Caprylhydroxamic acid draws less concern than methylisothiazolinone or parabens, ingredients tied to a jump in allergic reactions the past decade. Patch tests run by dermatologists and studies on real users both point to a better record for caprylhydroxamic acid. The Cosmetic Ingredient Review Expert Panel backs its safety—when used at the right levels.
Yet, not every skin story fits the textbook picture. I remember swapping to a “clean” serum, only to face itchy cheeks the next day. No parabens in sight, but there sat caprylhydroxamic acid low on the ingredient list. I later found out I’m in a small group whose skin can’t handle even this so-called mild pick.
No single rule covers everyone. Patch testing remains the best shield. Before using anything new, put a bit behind the ear or on the forearm, then wait two days. That small step helped me dodge red, flaky cheeks after the serum incident. Dermatologists recommend this method because reactions can be sneaky—sometimes days after trying a new formula.
Some experts believe more transparency could help. Product labels rarely explain the concentration used or warn about possible reactions in extra-sensitive skin. Brands could be clearer about the low but real chance of a problem. Formulators also experiment with plant-based extracts, but these can trigger responses in people with plant allergies. Sharing these facts with shoppers creates trust, not fear.
Caprylhydroxamic acid brings a solution for many but not all. Skincare’s personal, and ingredients—even the gentle ones—come with trade-offs. People deserve more than just a “safe for all” claim. With so many variables, the best step means learning skin’s quirks and not hesitating to ask what’s inside a favorite product. Skincare should feel good, not mysterious.
Caprylhydroxamic acid, often spotted on ingredient labels as CHA, has picked up momentum in personal care and cosmetics production. Known as a chelating agent and preservative, it blocks the growth of yeast, mold, and bacteria, which tend to spoil formulas quickly. Many manufacturers searching for alternatives to parabens and formaldehyde donors take a closer look at CHA because it works in oil-free and low-pH environments.
Sorting through various preservatives back in my years of working with indie skincare startups, I remember how hard it was to find something that balanced both safety and punch. The appeal for CHA often came up in discussions not just because of its clean profile, but because the market demanded more transparency around ingredients. With so many concerns about irritation and allergies linked to older preservative systems, brands moved fast to embrace new options that wouldn’t scare off savvy buyers.
People in product development offices talk a lot about hurdles—especially in the fight against contamination. Anyone formulating natural and “clean” products runs into these headaches: spoilage, unstable formulas, or microbiological growth. Preserving a blend often means leaning on multiple preservatives to cover a wider range of bugs and real-world conditions.
CHA brings strong antifungal power. By pairing it with other preservative agents such as phenoxyethanol or potassium sorbate, formulators stack their defenses, catching both bacteria and fungi. A formula with only CHA, for example, can face testing failures if bacteria levels overwhelm the acid’s core strength. I remember a few runs of an all-natural mask based on CHA failing lab benchmarks—until a small percentage of phenoxyethanol was added. Not every combination wins, but those that do tend to pass tests from start to shelf.
Practical chemistry matters as much as theory. Some preservatives in high concentrations irritate the skin or disrupt the smooth feel people expect from lotions and serums. Too many times, I have seen developers reach for single-ingredient solutions only to find they compromise either safety or sensory experience. Using lower levels of each preservative, paired intelligently, can help brands dodge these pitfalls and deliver safer, long-lasting formulas without harsh side effects.
Shoppers push back when they spot parabens or older preservatives in their creams or shampoos. Brands chase alternatives like CHA to hang their hat on “clean” labels, but every swap comes with risk. I learned this quickly working through rounds of preservative challenge tests, where one failed batch can delay a launch and pile up costs. Mixing CHA with a companion preservative, rather than leaning on it alone, helps smooth the path to market by covering more threats at lower individual doses.
Microbiology doesn’t cut corners. Factory floors, shipping trucks, steamy bathrooms—all breed conditions for microbes. Pairing CHA with partners like sodium benzoate or glyceryl caprylate creates hurdles at multiple stages. This mix mimics the complexity of the real world, where formulas see temperature swings, air exposure, and all sorts of environmental stressors before landing in someone’s routine.
Fear and misinformation flood ingredient debates. CHA, with a strong data package on irritation and low toxicity, brings peace of mind to consumers tired of red flag buzzwords. But “natural” and “safe” often clash in practice. Adding a partner preservative paves the way for more reliable protection, fewer recalls, and less risk of allergic flare-ups. Relying on a systems approach rather than a single hero ingredient helps shift the conversation toward evidence and away from hype—a lesson anyone invested in both consumer safety and business stability learns fast.
With a little chemistry know-how and real-world experience, mixing CHA with the right partners keeps both products and reputations in top shape.
Caprylhydroxamic acid makes its way onto more skincare labels these days, stepping in as a preservative when people want to avoid parabens. The reason for the shift comes from its ability to fight off bacteria and fungi, so it keeps serums, lotions, and creams fresh much longer. This new ingredient deserves some attention for what it adds and the risks it carries, since no chemical works in a vacuum.
Caprylhydroxamic acid comes from coconut or palm oil, but even plant-based chemicals can upset skin. The biggest concern comes from irritation. Some studies report that sensitive skin types sometimes flare up with redness, itching, or tingling after using products that include this acid. This comes from the molecular structure, which interrupts the life cycle of microbes. In the process, it can sometimes disrupt the delicate environment of the skin’s upper layer.
Personal experience backs up this research. People with eczema tell me that switching to “natural” or “paraben-free” formulas doesn’t always solve their irritation problems. Some notice burning sensations after a few days. Dermatologists in the field often point to the presence of caprylhydroxamic acid plus other acids or alcohols in the mix. These kinds of formulas can become too harsh when used daily or on broken skin.
Approaching this with some balance, caprylhydroxamic acid does its job better than most alternatives, mainly because it works even at low concentrations. Most products use it at levels of about 0.3% to 1%. At these levels, it usually does not cause widespread problems, at least for those with average skin. Allergic reactions seem rare. Still, someone who already struggles with fragile skin should pay extra attention. Patch testing a new product at home — applying a small amount to the inner arm or behind the ear — often reveals in advance whether a reaction will follow.
Human safety studies cover only so much ground, though. The cosmetics industry relies on data gathered from relatively healthy volunteers. People with compromised skin barriers, such as those living with chronic dermatitis, often react in ways the test groups do not. Kids and elderly people also carry higher risks.
No single chemical solves the preservative dilemma. Caprylhydroxamic acid works well, sometimes too well, and may team up with other chemicals to ramp up irritation. Before buying into any “safe,” “natural,” or “green” claim, it helps to read the ingredient list and understand your own skin history. Seeking unscented formulas and products with minimal added acids can make a difference for those who get rashes easily.
Looking for clearer regulations and truthful marketing remains important for consumers and clinicians alike. Manufacturers should provide better transparency on concentration and usage instructions. Dermatologists and pharmacists could help more by educating about less obvious triggers, not only the old standbys. Staying alert to new research, listening to personal reactions, and staying honest about side effects—these all help families and patients make better choices every time they open a new bottle of face cream.
Caprylhydroxamic acid pops up across plenty of skin care lines, and it earns its keep in formulas as a reliable preservative. This ingredient knocks out yeast and mold that threaten product safety. In daily practice, product formulators see it as a much-needed alternative to parabens and formaldehyde releasers, which have fallen out of favor with health-conscious shoppers.
With consumer demand for clean labels, brands hunt for preservatives that protect without stirring controversy. Caprylhydroxamic acid gives a strong answer by keeping things simple and effective. No artificial after-smell, no risky breakdown products, no question marks raised by dermatologists.
Most technical sheets and regulatory resources recommend caprylhydroxamic acid in the range of 0.3% to 0.5%. This level gives enough protection across a broad pH without causing formula issues or triggering irritation. Cosmetic chemists who have worked hands-on with this ingredient often stick to this guideline. Push too far above 0.5%, and it can bump up costs and sometimes increase chance of stickiness or tackiness, especially in lighter emulsions.
Many ingredient suppliers, including Inolex and Spec-Chem, back up these numbers with real challenge test results. In these tests, typical formulas passed microbial challenge with 0.3% included, even without parabens or phenoxyethanol backing them up. In my own experience running preservative screenings, hitting 0.5% brought extra protection but rarely solved a problem that 0.3% left behind.
Caprylhydroxamic acid almost always teams up with other preservatives — most commonly phenoxyethanol or potassium sorbate. This keeps costs in check and puts the brakes on microbial growth from several directions. It’s smart to consider the water content and expected shelf life. A face cream sitting on a bathroom shelf sees a lot more action from humidity and users’ hands than a tightly sealed serum.
In some cases, formulas with a lower or higher water content need a slight adjustment. For example, a lightweight gel might hold up fine at 0.25%, but a rich moisturizer or a formula stored in a jar (where many hands dip in) deserves at least 0.4%. These tweaks don’t always show up in technical papers, but seasoned formulators pick up on them from real-world stability and challenge tests. It pays to run extra challenge tests if formulas push the extremes of pH, as preservative action can change at acidic or alkaline levels.
The EU’s Cosmetic Regulation lets brands use caprylhydroxamic acid at up to 1%, though most brands steer well under that. Patch testing and clinical studies show that most people tolerate it even at maximum recommended strengths. Still, some users with ultra-sensitive skin can get redness when exposed to any preservative, so brands tend to pair it with soothing botanicals or use airtight packaging whenever possible.
As more shoppers read ingredient labels, product makers face increasing pressure to choose the right ingredients and levels. Caprylhydroxamic acid covers the bases without crossing any regulatory red lines, giving both brands and customers peace of mind.