Digging into the history of butanedioic acid gives a sense of how science moves from curiosity to utility. This dicarboxylic acid, also known as succinic acid, emerged from 16th-century explorations in amber distillation. Early chemists didn’t have the luxury of spectrometers or automated synthesis; they worked by observing crystals and sniffing out new compounds by hand. Industry picked up on butanedioic acid in the late 1800s as purification and large-scale production became possible. First tapped as an intermediate in dye manufacturing, its role steadily grew. By the 20th century, processes expanded thanks to improved fermentation and chemical synthesis techniques. Eventually, the food, pharmaceutical, and polymer industries recognized its value, and its production jumped in regions with plenty of biomass or petrochemical resources.
Talking about butanedioic acid means talking about a chemical with flexibility. It’s a white, odorless powder or colorless crystal, easily dissolved in water, ethanol, and other polar solvents. Industrial chemists see it as a building block, finding its way into everything from plasticizers to medicines. With annual global production spanning tons, companies rely on its predictable behavior. Some might find it sold as “succinic acid” or by trade names shaped for food regulations or industrial standards. Fine chemicals, specialty plastics, biodegradable polymers—there’s almost always a place for it when gentle acidity and reactivity are called for.
Butanedioic acid melts around 185°C and boils with decomposition at higher temperatures. Its density sits near 1.56 g/cm³. The molecule holds two carboxylic acid groups at each end of a four-carbon backbone, making it soluble in water but less so in non-polar solvents. pKa values of 4.2 and 5.6 signal moderate acidity. Structurally modest, this acid still plays hardball in redox and coupling reactions. Exposure to dry air keeps it stable for months on end, so long as it’s shielded from moisture and rough treatment.
Industry standards call for clear labeling and strict content specifications. Purity grades range by application: technical grades for industrial synthesis, food grades for additives with heavy scrutiny for contaminants and heavy metals, and pharmaceutical varieties carrying even tighter limits. Packaging info covers batch numbers, expiration dates, and recommended handling. The CAS number—succinct, nearly universal—makes tracking and purchasing a breeze for buyers and regulators. Safety panels warn about inhalation risks and encourage storage in cool, dry places. Labeling never overlooks acute exposure or fire data, especially with strict GHS (Globally Harmonized System) guidelines.
Industrial preparation splits into two main camps: petrochemical synthesis and biotechnological fermentation. The chemical route often cracks maleic anhydride or n-butane with catalysts, yielding butanedioic acid efficiently on scale. This process ties the industry to fossil feedstocks. Growing pressure for sustainability gave rise to bio-based production using genetically engineered microbes. Corn glucose or other renewable carbohydrates become feedstocks, and fermentation in large reactors reaps succinic acid cleanly. Filtration, crystallization, and drying follow. This bioprocess wins fans in the push for green chemistry, offering lower emissions and a lighter environmental footprint.
Butanedioic acid pulls its weight in chemical synthesis. Its two acidic protons enable salt formation with bases like sodium or potassium hydroxide, bringing about succinates. Condensation reactions with alcohols roll out esters used as plasticizers. Under strong heating, dehydration yields succinic anhydride, a versatile intermediate for further reactions. Hydrogenation morphs it into 1,4-butanediol, an ingredient for spandex and polyesters. Its ability to couple with amines carves out a path for biodegradable polyamides, making it a darling of the bioplastics world. Redox reaction versatility even sets the stage for pharmaceutical and agrochemical syntheses.
This chemical often answers to more than one name. "Succinic acid" dominates textbooks and regulatory filings, tracing back to the Latin root for 'amber.' Chemical registries and procurement lists sometimes include butanedioic acid, ethane-1,2-dicarboxylic acid, or E363 in the case of food labeling. Trade catalogues adapt branding for various application markets—sometimes fine-tuning labeling for food, sometimes for technical audiences. This versatility in naming never muddies regulatory tracking, as CAS number 110-15-6 stays glued to every shipment and invoice.
Despite its gentle reputation in food and pharma, butanedioic acid needs careful handling. Dust exposure in manufacturing settings can cause mild irritation to skin and mucous membranes. Inhalation by workers demands decent ventilation, and personal protective equipment remains non-negotiable in active plants. U.S. OSHA and EU REACH regulations both spell out exposure limits, proper disposal, and emergency responses. Spill cleanups rely on dry methods to avoid messy acid-base neutralization. Fire risk stays low, but safety sheets ban mixing with strong oxidizers or handling near open flames. Workers go by protocols that cover not just exposure but storage and waste management, ensuring both human and environmental safety.
Butanedioic acid pops up in more places than most might expect. Food technologists count on it as an acidity regulator—labelled E363—picking up the slack in soft drinks, jams, and gelatin desserts. Pharmaceutical industries tuck it into certain antibiotics and vitamins, where predictable solubility routines improve drug delivery and stability. The chemical also feeds into leather tanning, electroplating baths, and specialized detergents. In the realm of polymers, succinic acid underpins the push toward bio-based plastics. Polybutylene succinate (PBS) now shows up in compostable packaging and molded goods, giving plastic makers a greener alternative without sacrificing durability. The ability to pair with diverse partners—alcohols, amines, glycols—broadens its footprint each year.
Research never sleeps on butanedioic acid. Synthetic chemists chase more efficient catalysts for conventional production, cutting energy demands and boosting conversion rates. Microbiologists tinker with yeast and bacteria genetics, chasing strains that churn out higher yields from agricultural waste. Circular economy advocates search for routes to recycle spent succinic acid or capture it from carbon-rich exhaust streams. In materials science, teams work on tuning the toughness and degradability of PBS blends for everything from medical implants to 3D printing filaments. Green chemistry benchmarks put pressure on solvent use and emissions at every stage, forcing labs and plants to balance cost, process simplicity, and environmental impact.
Toxicologists have kept a close eye on butanedioic acid, especially in food and pharmaceutical contexts. So far, studies agree that it comes with low acute toxicity. Oral exposure passes through the human body’s metabolic pathways—breaking down into carbon dioxide and water via the Krebs cycle, a route the body already knows well. High concentrations do cause local irritation and gut discomfort. Chronic exposure studies in animals show limited long-term risk at normal environmental and dietary levels. Regulatory agencies like the U.S. FDA and European Food Safety Authority classified it as Generally Recognized As Safe (GRAS) for limited uses. Even so, ongoing research keeps tabs on occupational exposures, potential impurities, and environmental effects of widespread use or breakdown products in soil and water.
Looking at the future, butanedioic acid stands on the front line of sustainable chemistry. As manufacturing pivots away from fossil resources, demand for bio-based acids and green polymer intermediates climbs. Crop residues, industrial CO2 waste, and even municipal biomass can nourish production strains that spit out succinic acid. The rise of plastic bans and consumer interest in compostable goods lines up with broad investment in PBS and similar materials. Pharmaceutical routes also push research into chirality and enantiomerically pure compounds, areas where tailored synthesis from bio-based intermediates could tip the scales in drug design. Markets in Asia and North America eye further capacity expansion, pulling farmers, biotechnologists, and chemical engineers into new alliances. As global economies target net-zero emissions, the versatility and biodegradability of butanedioic acid promise a bigger role not just in industry but in closing the loop against chemical waste and pollution.
Butanedioic acid is better known in science labs as succinic acid. Many never hear its name outside a chemistry class, yet products in supermarkets and medicine cabinets wouldn’t work the same without it. This simple compound, found in sugar cane, beet juice, and even amber, has a range of uses grown out of both nature and innovation. Succinic acid has become a valuable ingredient across industries because it lends itself naturally to so many processes. I once watched a friend in food science turn it into a tart flavor agent, giving a punch to certain candies without leaving the odd aftertaste some other acids leave behind.
Baking, beverage, and snack companies often look for ways to enhance taste without breaking the bank. Succinic acid fits that bill. It acts as an acidity regulator in candies, sauces, and even jams. In the snack aisle, a bit of succinic acid can bring out sour notes when used alongside citric acid. The food industry also uses it as a preservative, adding a layer of defense against spoilage. Safety data over the years supports its use, and federal agencies including the US Food and Drug Administration give it a nod as generally recognized as safe (GRAS). Knowing that scientists back its safety makes it easier to trust strange-sounding ingredients in snacks or soda.
Doctors and pharmacists look for active ingredients that treat, buffer, or help absorb medicine in the body. Succinic acid plays a supporting role in some migraine medications and vitamin formulas. In tablets and capsules, it helps buffer pH, making sure the mix stays stable. Research teams explore succinic acid’s potential in treating inflammatory diseases, and injectable forms sometimes help stimulate metabolism, according to leading pharmaceutical journals. Its antioxidant qualities even push researchers to test its abilities to protect cells from oxidative damage. These applications show a natural compound can step up to help meet tough medical needs.
Plastic production gets a boost from succinic acid, especially as companies overhaul manufacturing for a greener planet. Plant-based succinic acid now acts as a building block for biodegradable plastics and polyesters. Not having to rely on petroleum reduces carbon footprint. I’ve seen local start-ups pivot to these bio-based alternatives, hoping to meet stricter climate regulations. Farmers use succinic acid too, mixing it into plant fertilizers to promote healthy growth. This keeps crops more resistant to stress factors and drought, an advantage in regions struggling with unpredictable weather patterns.
As the world hustles toward sustainable solutions, the demand for bio-based succinic acid grows. Most production today relies on fermentation—using microbes to turn sugars or agricultural waste into succinic acid. This lowers the environmental burden, but scaling up isn’t straightforward. Factories face challenges sourcing enough renewable feedstock and energy. More investment in innovative biotech may help. Giving smaller businesses easier access to affordable succinic acid could help drive both local industry and environmental improvement. Pressure from global markets could push bigger manufacturers to clean up their production, but government action often comes slowly.
Succinic acid may sound like something only lab techs should worry about, but it keeps surfacing in life’s daily details—from flavor in a favorite snack to reducing waste in manufacturing. The need for sustainable, safe ingredients becomes more urgent each year. Backed by years of lab research and clear health records, butanedioic acid proves that helpful and harmless ingredients do exist, sometimes hiding under unfamiliar names.
Butanedioic acid, which folks in the lab often call succinic acid, shows up as a white, odorless powder. You wouldn’t look at it and think “hazard,” but safety doesn’t always scream for attention. Anyone who’s spent time working with chemicals, even the ones labeled “moderate hazard,” knows that accidents can show up when you least expect them. Succinic acid can irritate your skin, eyes, and lungs. Breathing in the dust or having it rub into a cut makes for a rough day.
For years, people have told new lab workers that gloves aren't just for show. Nitrile gloves block direct skin contact and stop the powder from finding its way through cuts or hangnails. Lab coats and eye protection aren’t optional, even if they feel awkward or warm. I remember a situation where a student in the lab refused eye goggles because he thought a little powder couldn't hurt. It only took one accident with a small splash to change his mind—his eyes stung for hours, and luckily no lasting harm followed.
Keeping the chemical out of sunlight helps it stay stable. A solid, sealed container stops moisture and air from turning the powder into clumps. Nobody wants to be the person who tries breaking apart a cake of chemical with a spatula, sending dust up into the air. If you spill some, don’t just sweep it away with your hand. Grab a mask, toss on the gloves, and gently scoop it up with damp paper towels or a spatula. Simple routines like this prevent trouble in shared spaces.
The smell might not punch you in the nose, but the dust can still float around. Using a fume hood or working by an open window goes far to keep the air clear. I’ve been in labs with no proper airflow, and itchy eyes plus a scratchy throat always followed. The most careful technique in the world won't make up for bad ventilation. Setting up extractor fans saves headaches for everyone down the line.
Washing up with soap after handling butanedioic acid seems simple but stops accidental contamination. Eating lunch or rubbing your eyes after handling chemicals is the quickest way to turn a safe day into a visit to the nurse. All bottles need clear labeling—nobody enjoys the panic of an unlabeled white powder in the fridge. Proper labels help protect everyone who shares the bench, not just the person who put the powder there.
Nothing beats solid, hands-on training. Reading a Material Safety Data Sheet only goes so far. Watching someone demonstrate safe transfer techniques sticks with you much longer than dry instructions ever will. Even after years in a chemistry lab, I double-check the method when new students ask how to dissolve or weigh a powder. Teaching others and keeping each other in check keeps everyone safer. Mistakes still happen, but teams that talk about safety catch small problems before they become emergencies.
Between spills, splashes, and accidental inhalation, things can go sideways. Emergency showers, eye wash stations, and first aid kits should stay close to busy workspaces. If some powder gets in the eye, rinse with water for at least fifteen minutes, then head straight to the doctor. If skin turns red, wash with soap and water and keep an eye on it. For bigger problems, get medical help right away. Having these stations nearby has saved quite a few folks from more serious injury.
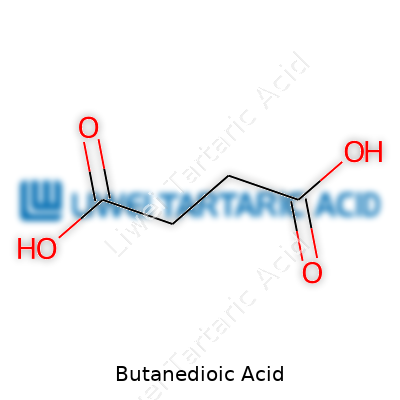
Butanedioic acid brings to mind a name you might recall from high school chemistry: succinic acid. Its chemical formula is C4H6O4. Scientists and industrial workers see this compound regularly, popping up in everything from food additives to specialty polymers. If you walk through a chemistry lab or a food processing plant, you’re just as likely to come across containers labeled with its alternative name — succinic acid — as you are to stumble on jars of citric acid or table salt.
The precise arrangement behind butanedioic acid’s capabilities lies in its structure: four carbons, six hydrogens, and four oxygens. That makes each molecule pack a molecular weight of 118.09 g/mol. The neatness of its formula hints at versatility. Two carboxyl groups tethered across a four-carbon chain, with hydrogens and oxygens on duty for chemical balance. Structurally simple, yes — but powerful in real-world uses.
Working in food production, you get to know which acids shape flavors, preserve goods, and lend that sharpness to your favorite sour candies. Butanedioic acid is behind the sour note of some fermented foods, and it helps control acidity in processed snacks. My time collaborating with food technicians showed me how a pinch of this acid contributes just enough tang without overwhelming the palate.
Biomedical researchers harness its anti-inflammatory and antioxidant properties. Succinic acid shows up in supplements and topical treatments thanks to claims around cell metabolism and skin brightening. Scientific publications highlight studies where it reduces swelling or supports energy production, building on decades of research into metabolic cycles. That cycle — the citric acid cycle or Krebs cycle — features succinic acid front and center, moving energy through living cells in everything from bread mold to blue whales.
Butanedioic acid plays a significant part in moving chemistry toward a cleaner, greener era. Traditionally, companies made it with fossil fuels. Now, fermentation processes using renewable sugars, agriwaste, or even carbon dioxide are gaining ground. Fermentation brings its own challenges: impurities, inconsistent yields, costs that fluctuate with crop harvests. Yet, labs from California to Singapore keep improving yields and cutting waste, underlining the economic pull behind sustainable methods.
Compounds like succinic acid are prized for making biodegradable plastics. Research points to bioplastics as a credible alternative to petroleum-based products, slashing carbon footprints while delivering similar toughness and flexibility. Companies face hurdles scaling up these green processes, but the track record continues to improve.
Food and drug regulations lay down strict standards for raw materials like butanedioic acid. Purity matters — impurities slip in during production and can affect product safety. Technicians spend hours running tests like chromatography and titration to hit quality benchmarks. International guidelines, such as those from the U.S. Food and Drug Administration and European Food Safety Authority, guide acceptable levels. I’ve seen firsthand how regulatory compliance enables trust, letting manufacturers develop safer, more innovative products.
Scaling up sustainable production and maintaining cost-effectiveness presents the toughest challenge. Researchers are experimenting with genetically engineered microbes that promise faster, cleaner conversion from plant sugars. Some pilot projects aim to use agricultural waste streams that would otherwise end up in a landfill. Continued collaboration between academia and industry drives both innovation and price reduction, making eco-friendly butanedioic acid more widely available for everyday goods.
People who handle chemicals often overlook the basics. Years ago, working in a small research lab, I watched a colleague clean up a leak caused by improper storage. The container, tucked beside a heat source, developed a crack and released fumes. Butanedioic acid, better known by some as succinic acid, doesn’t carry the same reputation as stronger acids or solvents. Even so, no one should underestimate it. If handled carelessly, risks jump from harmless spills to real health threats.
Succinic acid tends to turn clumpy and degrade if exposed to constant moisture. Humidity creeps in easily through loose lids and suspect seals. Once, a batch left open during a humid summer lost its granular texture and gummed up lab equipment. The damage cut into research time and budgets. Dry conditions matter. Proper storage containers with tight seals stop this compound drawing in moisture and clumping. Avoiding glass jars with worn-out rubber gaskets saves headaches later.
You won’t find the best chemical storage beside a lab sink or any area prone to splashes or leaks. Setting butanedioic acid near water invites release or dilution. Accidental slips get more likely in cramped or cluttered supply closets. Staff with limited space tend to shove bottles wherever they fit, but this increases the chance of mixing incompatible substances. Dedicated shelves, clearly labeled, prevent that chaos. After watching cross-contamination lead to ruined test runs and wasted hours, keeping acids separate becomes second nature.
Succinic acid usually stays solid at room temperature, but the story changes with swings in heat. Too much warmth, such as what you get near radiators or sunny windowsills, speeds up decomposition. I’ve seen what a month of summer heat can do to a neglected supply room—sticky residue and warped labels everywhere. Reliable storage requires cool, shaded locations, out of direct sunlight. Facilities with air conditioning or even simple fans help stop the temperature from swinging too far.
After working in both large warehouses and school chemistry storerooms, one thing never gets old: safety equipment saves skin and lungs. Succinic acid irritates eyes, throat, and mucous membranes. Accidental spills happen more often than most care to admit, especially during late-night experiments or busy production runs. Rubber gloves, goggles, and long-sleeved lab coats ward off minor injuries. Emergency eyewash stations help, but no one wants to use them. Setting up a clear protocol—and keeping protective gear within reach—carries more weight than any MSDS sheet can explain.
Most people think about the immediate. In my time managing chemical stocks, subtle mishaps—like a drip running down a jar or a screw cap that never quite fit right—slowly corroded metal shelving. Before long, shelves buckled, and unnoticed crusts formed that exposed custodial staff to chemical traces. Plastic or coated shelving stands up much better over time. Quarterly inspections, written logs, and record-keeping force a double-check on storage conditions. My team caught leaks earlier and prevented costly damage down the road.
Supply rooms often evolve from makeshift beginnings. Retrofitting racks, labeling bins, and dedicating floor space never happens overnight. Still, every mistake tells a story. Workers need ongoing training, not just a one-time orientation. Supervisors need to schedule checks instead of hoping for the best. In the end, safe storage of butanedioic acid isn’t complicated if you learn from past errors and combine a little common sense with practical safeguards.
People hear “butanedioic acid” and the name alone grabs attention. It’s better known by its more familiar name: succinic acid. Folks working in manufacturing, food, and even agriculture all bump into this stuff. Succinic acid pops up as a preservative, acidity regulator, and sometimes as a building block for bioplastics. Its root comes from plants and even some fermentation processes, so folks see it as a big step away from the footprint left by petrochemicals. Still, that doesn’t mean the story stops with “plant-based equals harmless.”
Take a closer look at the way industries use succinic acid and things don’t seem that alarming. The acid breaks down quickly in air and water, and doesn’t hang around for a long time. Intense exposure isn’t likely outside of industrial facilities, and those places usually keep a close eye on discharge and air quality. In small amounts, the stuff isn’t toxic to people, animals, or fish. The U.S. Environmental Protection Agency ranks it pretty low on the risk scale. That’s not license for carelessness. In my own experience working at a food production plant, we handled barrels of organic acids like succinic acid. Safety data sheets rarely flagged it for long-term environmental threat. Spills demanded routine caution: goggles, gloves, quick cleanup. Rinsed down the drain and diluted, it didn’t spark any panic.
Not every aspect of production goes easy on the planet. Making succinic acid from fossil fuels chews through a lot of energy and spits out greenhouse gases. Recent methods lean into using sugar or even agricultural waste, where bacteria chew on plant matter to pump out the acid. This “biobased” fermentation kicks fossil fuels to the curb, and that’s made a real difference. Carbon emissions can plummet as much as 90% using sustainable feedstocks. These new ways pull the chemistry world toward more responsible manufacturing.
Risks climb if big, concentrated spills hit waterways before getting neutralized. Stunned aquatic life and dropped pH levels can happen with anything acidic, not just butanedioic acid. Realistically, accidental spills don’t come close to the scale of damage seen with pesticides or heavy metals. Waste plants dilute it so much, it loses the bite before exiting into rivers. The basic facts say this compound prefers to break apart fast, especially under sunlight and plenty of oxygen. Runaway environmental disasters from succinic acid look more like movie script than reality.
Experience teaches that blind faith in “natural” chemicals does more harm than good. Plant-based or not, anything tossed into the world in huge chunks can come back to bite. Keeping an eye on how much acid leaves factories, updating safety practices, and sticking to green production mean less worry for everyone. Wastewater treatment plants need the tools and support to manage all sorts of inputs, including the odd organic acid. Anyone making or using these chemicals owes it to the community to keep accidents rare and plans updated.
Plenty of substances cause more alarm bells than butanedioic acid. Chemicals with long life spans and toxic byproducts overwhelm ecosystems and get headlines. Succinic acid, when handled responsibly, earns a low spot on the hazard list. The best way forward: keep pushing for sustainable production, mind the local environment, stay trained, and never skip the basics. Most days, nature cleans up after small mistakes faster than news headlines can keep up.