Centuries ago, European apothecaries blended minerals and organic extracts to create remedies both magical and faulty. Antimony potassium tartrate—sometimes dubbed "tartar emetic"—was born out of efforts to treat fevers, regulate bowels, and evoke vomiting. This compound didn't hide in shadowy corners of history; prominent physicians of the 17th and 18th centuries prescribed it with earnest hopes, not always knowing the risks. Anyone who reads those records can't help but feel a mix of awe and unease. French chemists like Jean-Baptiste Dumas worked to clarify its structure once tools for chemical analysis matured. Later, its medicinal use waned, but veterinary medicine and chemical industry saw reason to keep it around. There's real human error woven through its journey. Enthusiasm for a miracle cure faded as poisonings piled up, yet its unique chemistry survived the trials of time.
Antimony potassium tartrate serves today as more than a curious medical relic. Industry puts it on the payroll: textile dyeing, leather tanning, and as a mordant for fixing dyes onto fabrics. The compound lands in many laboratories as a catalyst for synthetic reactions. Chemists see it as a double salt—a meeting of antimony, potassium, and tartaric acid. This precise blend grants properties that pure antimony salts don't offer alone. Its presence in chemical test kits, notably for detecting reducing sugars or peroxides, keeps it relevant long after its time in clinical medicine ended.
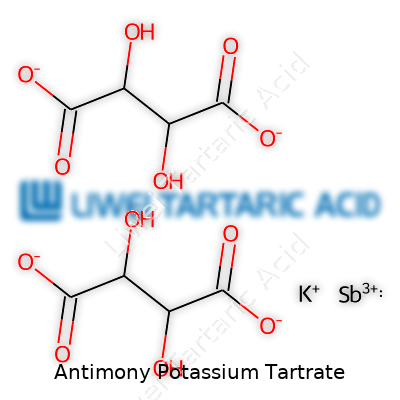
Typical samples of antimony potassium tartrate appear as colorless, crystalline powders. They dissolve fairly easily in water, creating a slightly acidic solution. When stored in dry air, the compound remains stable. Humidity, on the other hand, leads to slow decomposition and can reduce shelf life. Molecular formula reads as K2Sb2(C4H2O6)2·3H2O, showing its complexity. Laboratory experience shows the bitter metallic taste, and inhaling the powder even by accident is never advised. Its density runs close to 2.6 g/cm3, and decomposition on heating produces toxic antimony fumes.
Manufacturers stamp the packaging with the batch number, purity, manufacturing date, warnings, and storage directions. A bottle in my lab features hazard pictograms: corrosion, toxicity, and environmental hazard. The label spells out antimony potassium tartrate trihydrate, commonly sold at analytical grade with at least 99% purity. Quality control tests for sodium, lead, and arsenic ensure these impurities remain far below allowable thresholds. Transport and disposal notes take up half a page—certain countries demand more detail, reflecting concern about what happens long after the bottle gets opened.
Synthesis often starts with antimony trioxide or antimony oxide, reacted in a heated solution with potassium hydrogen tartrate. Controlled pH and temperature dictate whether a clean crystalline product falls out or problematic byproducts muddy the process. After thorough filtration and slow evaporation, crystals form and get rinsed with cold water to remove excess reactants. Proper technique matters; inexperience invites yield loss or excessive impurities. Each batch feels like a chemistry lesson, testing timing and patience. Skilled technicians avoid shortcuts, knowing contamination leads to poor outcomes and future safety risks.
Antimony potassium tartrate reacts with strong mineral acids, leading to decomposition—antimony precipitates as a white powder. Alkalis, on the other hand, break it down to antimonate and related species. Heating leads to rapid decomposition, releasing antimony oxides and potentially dangerous gases. Organic reductions reduce the compound slowly, and laboratory experience proves it interacts with many reducing agents. Titration against standard reducing solutions—think Fe(II) or SO2—still plays a role in certain chemistry exercises.
In older texts and niche catalogs, antimony potassium tartrate picks up many aliases: tartar emetic, potassium antimonyltartrate, emetic tartar, and antimonyl potassium tartrate. Trade names sometimes hide the true identity, increasing risk in the hands of the untrained. The IUPAC name remains potassium antimony(III) tartrate trihydrate, but few people outside advanced chemistry classes use the full version. When teaching, it’s best to explain each synonym, since product labeling often varies based on region or supplier.
Few chemicals in the lab command as much respect for toxicity as antimony salts. Eyes and skin demand proper protective gear—goggles, gloves, and sometimes a full face shield. Fume hoods aren't optional when handling powders or solutions. Ingestion or inhalation can trigger violent vomiting, diarrhea, irregular heartbeat, and systems collapse. Even small spills create cleanup emergencies, as antimony compounds persist in the environment and accumulate in the food chain. Regulatory standards from OSHA and the European Chemicals Agency require training, spill protocols, and exposure monitoring. Practical experience taught me to avoid casual familiarity—it only takes one careless moment to create a crisis.
Antimony potassium tartrate lost its seat as a medicine, and with good reason; safer alternatives replaced it for most clinical purposes. Yet its role as a catalyst for polyester synthesis, especially in large-scale fiber production, shows its industrial relevance. Textile workers, chemists, and manufacturers encounter the compound more often than doctors do today. In the realm of analytical chemistry, it assists in detecting glucose and in titration protocols for hydrogen peroxide. Modern applications in veterinary medicine linger in places where alternatives haven't taken over. A few niche uses remain in ceramics and pigments, where its chemical stability brings unique results.
Chemists and toxicologists pursue alternatives that retain the useful properties without the risks. In academic research, the compound offers lessons in complex salt formation, stereochemistry of tartrate ligands, and redox reactions. Ongoing papers look for ways to degrade antimony compounds in wastewater or to recycle them from spent catalysts. Some industrial laboratories seek process innovations to limit worker exposure and cut down on environmental release. Green chemistry movements pressure industries to shift, but old processes often die hard. Each incremental improvement in safety or yield builds on lessons from experiments—both successful and failed.
Animal studies confirm the reputation: antimony potassium tartrate is a strong emetic and a cumulative poison. Chronic exposure builds up antimony in organs like the liver, heart, and spleen. Studies on industrial workers document increased rates of respiratory diseases, cardiac issues, and digestive problems. Acute symptoms can appear in minutes: metallic taste, vomiting, cramps, muscle pain. Regulatory agencies in Europe and North America restrict occupational exposure sharply, but regions with loose enforcement see more accidents. Recent toxicology research focuses on mechanisms of cellular injury and long-term carcinogenicity. In my own work, careful tracking of exposure routes—dermal, inhalation, and ingestion—remains a non-negotiable requirement.
Antimony potassium tartrate walks a narrow line between usefulness and danger. Demand for it as a functional catalyst could fade as greener alternatives and less toxic compounds replace it in large industries. Yet in analytical labs and advanced chemical education, its unique properties may keep it relevant for decades. Researchers already investigate antimony-free catalysts for fiber production. Environmental science teams search for remediation strategies to clean up spills and contaminated sites. Over time, regulatory pressures will only increase, favoring chemicals with minimal toxicity. Product innovation will probably shift investment elsewhere, but history shows that unique chemicals rarely vanish completely. Anyone working with antimony salts carries the lessons of both their risks and their value forward, seeking safer and smarter ways to shape tomorrow’s industry and laboratory practice.
Antimony potassium tartrate, sometimes called tartar emetic, pops up in places most people don’t expect. The compound once played a role in medicine, industry, and even the lab, but its reputation now carries plenty of baggage. My own experience involved chemistry classes at university, where we learned its formula and properties, but saw only warnings about its handling and health hazards.
Decades ago, doctors prescribed this substance to treat parasitic diseases—Schistosomiasis and Leishmaniasis, both of which can ravage the human body. People sometimes forget that in the 19th and early 20th centuries, options for treating such illnesses barely existed. Having a compound that could suppress deadly parasites changed lives. Researchers found it worked by interfering with metabolic functions in the parasites, buying patients time against otherwise fatal infections.
One thing sticks with me from reading old pharmaceutical texts: physicians knew about the risk of side effects, but options were limited. Stories from this era highlight a time when the choice boiled down to risk and survival. That all changed once safer drugs replaced it. Nowadays, I rarely come across any modern recommendation for antimony potassium tartrate in healthcare. In most developed countries, its medical use has vanished, replaced by newer, gentler therapies.
Industry still uses this compound, mostly behind the scenes. It acts as a catalyst for certain chemical reactions—making adhesives, producing textiles like synthetic polyester, and acting as a mordant in dyeing. The food and beverage industry used to bring it in for winemaking, helping clarify wines and reduce unwanted color. I once toured a small winery in Europe where an older worker recounted adding this “emetic salt” years ago before regulations tightened and new methods came along. Today, most large-scale producers avoid these old ways. Regulatory agencies, spotting potential toxicity, set strict limits or outright banned the additive in foods and wines.
I’ve never handled this substance outside strict lab conditions, and there’s good reason. It’s toxic, and even small doses can bring up nausea, vomiting, and dangerous disturbances in the heart and liver. Large exposures can kill, a fact reflected in medical case reports over the centuries. Regulations from agencies like OSHA and EPA in the US flag the need for protective equipment and prevention of spills. Evidence points to chronic antimony exposure causing trouble for factory workers and communities living near industrial sources.
With its health risks and availability of safer alternatives, few modern professionals choose antimony potassium tartrate unless no substitute will do. Industry continues leaning on green chemistry initiatives, reducing or eliminating hazardous chemicals. Curriculums use antimony potassium tartrate as a cautionary tale, teaching new scientists the price of progress when safety falls behind. Research into catalysts and medical therapies keeps moving, reducing the need for old, dangerous chemicals like this one. Replacing high-risk substances with safer ones builds trust and protects public health, both in the lab and beyond.
Antimony potassium tartrate sounds like one of those complicated names from a high school chemistry test. People curious about whether this substance is toxic or hazardous often look for real evidence, not just textbook jargon. I remember a teacher handling old chemicals with more caution than curiosity, and antimony compounds were high on his worry list. This caution has merit.
This chemical, sometimes called tartar emetic, carries a legacy. It used to play a role in medicine, sneaking into treatments as a remedy for some illnesses hundreds of years ago. Back then, folks didn’t realize how dangerous repeated exposure could be. Nowadays, toxicologists are pretty explicit. Antimony potassium tartrate can harm you, especially if you breathe in the dust, swallow even a small amount, or get it on your skin.
Antimony, the key metal here, carries a toxic punch. According to the World Health Organization, repeated contact or ingestion can harm vital organs, and even tiny doses can kill. Symptoms after taking in antimony potassium tartrate include vomiting, diarrhea, trouble breathing, and heart issues. Even short-term exposure in a poorly ventilated lab can send someone to the emergency room. Lab workers and anyone handling this compound must wear proper equipment — gloves, goggles, and a robust mask aren't optional.
Regulators don’t take this substance lightly. The Environmental Protection Agency lists antimony as a hazardous element, linking chronic exposure to skin irritation, lung effects, and possibly cancer. The CDC and OSHA both set strict exposure limits for antimony compounds, including this tartar emetic. The material safety data sheet isn’t bedtime reading. Some key recommendations: keep it away from food, double-check the laboratory ventilation, and never use kitchen measuring tools to handle it.
Disposal also matters. Pouring leftovers down the drain spells trouble for the environment. Waste containing antimony demands hazardous waste procedures–sealed containers, careful labeling, and a call to certified disposal experts. Even transporting sealed materials triggers regulations, as this chemical can’t go through ordinary mail services.
Having worked with various chemicals, I’ve seen some folks underestimate compounds just because they’re not radioactive or bright green in color. The clear crystals of antimony potassium tartrate look harmless at first glance. Danger doesn’t always come with a warning sign. Chemical burns or accidental ingestion don’t just happen in careless labs; one slip or unsupervised moment at home ruins lives.
Teachers, hobbyists, and anyone in science outreach need clear warning messages. Training young students or amateur chemists about safe handling can save lives. It’s easy to let focus wander or skip safety glasses “just this once.” But with chemicals like these, even half a gram can lead to a hospital visit.
Better communication about hazardous compounds makes a difference. Mandatory labeling, robust science education, and easily accessible safety data help prevent tragedy. Some research labs have shifted to less hazardous substitutes for demonstration purposes. These days, public science workshops avoid bringing in such chemicals, preferring safe alternatives.
Antimony potassium tartrate teaches a broader lesson. Respect the risk, never underestimate a substance based on looks or outdated stories, and push organizations to keep updating safety protocols. The knowledge safeguard is just as important as any lab coat or glove.
Plenty of people work with chemicals every day, but Antimony Potassium Tartrate deserves extra attention. This compound shows up in labs, certain manufacturing settings, and even some veterinary practices, but it can turn problematic if handled carelessly. Health authorities have linked it to skin irritation, lung issues, and more if inhaled or touched, so responsible storage and handling are not just checkboxes for compliance—they protect those doing the work and everyone nearby.
This chemical comes as a colorless or white crystalline powder and doesn’t do well around moisture or extreme heat. Left exposed to air, it can start breaking down. Even a clean, neatly labeled storage container can’t fix problems after water gets involved. That risk of breakdown raises health and safety concerns since toxic dust or fumes could form and spread quickly. Keeping powders sealed in airtight containers, stored far from any sinks or steam pipes, is a smart start. Metal or hard plastic lidded tubs work well, sitting on shelves at chest height to avoid spills or splashes into the face.
Heat speeds up trouble. A warehouse or lab with a stuffy back corner gets a heat buildup, and with that comes risk. Ordinary room temperature is best—ideally under 25°C (77°F)—so a shaded storage space, shielded from sunbeams and far from heaters, wins every time. In one lab I shared, a simple thermometer hanging beside storage brought peace of mind. People catch problems early and fix HVAC slips before they get out of hand.
Bare storage meets only half the need. Working with this chemical calls for well-ventilated rooms, hoods, or extractors. Dust inhalation doesn’t just feel unpleasant—it can trigger serious respiratory symptoms. For jobs that involve weighing, pouring, or mixing, a local exhaust hood or even a small room outfitted with fans becomes a basic requirement, not a bonus. The difference shows up in fewer complaints, less coughing, and less dust settling onto work benches. That’s lived evidence that air flow makes a real difference.
Nothing replaces gloves, goggles, and a mask or respirator when handling this powder. I once skipped goggles only to have minor irritation remind me of the lapse for the rest of the day. Chemical-resistant gloves and long sleeves protect against splashes or accidental contact, which happens more often than anyone likes to admit. For repeated work, overalls or a lab coat keep clothing free from dust, reducing risk of exposure outside the workspace. Easy access to eyewash stations and showers should be non-negotiable in any facility using this compound.
Spills—and even suspected spills—call for calm and speed. No sweep-and-go with a regular broom: use a vacuum with a HEPA filter, or carefully dampen the area with a wet wipe, moving slowly to avoid sending dust airborne. Bags meant for hazardous chemical waste hold the debris until licensed specialists can remove it. Training everybody on the team to spot, report, and respond to spills turns messy situations into manageable ones.
People working with Antimony Potassium Tartrate keep these habits alive with ongoing training, clear signage, and open communication. Regular drills make sure folks remember how to act fast if something goes wrong. In my experience, a workplace that encourages questions and double-checks cuts accidents and keeps people healthier in the long run. Safe storage and handling turn a risky chemical into a manageable daily reality, and that changes lives for the better.
Antimony potassium tartrate, with the chemical formula K2Sb2(C4H2O6)2 · 3H2O, stands out not just because of its name but its distinctive makeup. In any standard lab, you’d spot it as a crystalline solid—almost always colorless or colorless with a slight sheen. Sometimes folks call it emetic tartar, a throwback to times when it played a part in medicine. Pick up a beaker of this stuff and you’ll notice those shiny crystals stacked together, almost like sugar but denser and with a faintly metallic hint, a dead giveaway you’re not dealing with anything sweet.
Its story runs pretty deep. This compound earned its stripes in history as an emetic and treatment for parasitic diseases, mainly leishmaniasis and schistosomiasis. Sure, newer and safer medicines shifted the healthcare scene, but antimony potassium tartrate made a mark. In my chemistry days, teachers hammered home the risks of using heavy-metal compounds, and this one’s on the list. You needed to pay attention, not just because of its toxicity, but since it showed how science learns from its mistakes.
Beyond medicine, antimony potassium tartrate crops up in other places too. Analytical chemistry labs often use it as a reagent, especially in the classic Marsh test for arsenic. It helps distinguish what’s safe and what’s not, which tells you why regulatory bodies like OSHA and NIOSH set exposure limits for handling this material. These strict guidelines exist because, like many heavy-metal salts, this compound can turn dangerous with just a bit too much exposure. I’ve seen labs stress the importance of gloves and proper ventilation, not as a formality but as a lifeline.
Antimony potassium tartrate proves that science and safety always belong together. Cases of accidental poisoning in the past drove reforms in lab protocols. Any professional who’s catalogued hazardous materials knows not to underestimate antimony compounds. Short-term symptoms, including vomiting and muscle cramps, remind us that even historic drugs come with steep risks. Looking back, we see the necessity in pushing for non-toxic alternatives wherever possible. Chemists today often reach for safer reagents and practice rigorous waste disposal, spurred on by lessons learned from compounds like this one.
Water contamination by antimony compounds acts as a red flag in some communities. The EPA set a maximum contaminant level for antimony in drinking water, only 6 parts per billion. That small figure speaks to the importance of monitoring labs and factories, holding them to high standards to protect both workers and public health. Regular audits and transparent reporting build trust and keep people safe.
Science never stands still, and understanding chemicals like antimony potassium tartrate means more than memorizing facts. My own lab stints taught me the importance of awareness and honest communication, especially for substances with checkered histories. By translating complex chemistry into practical advice, the whole field not only honors the past but secures a safer future. Listening to seasoned professionals, absorbing real-world stories, and following evidence-based guidelines offer far more than just formulas and appearances.
Antimony potassium tartrate isn’t a household name, but people in labs and certain industries know it well. It’s useful for research and manufacturing, but its dangers don’t disappear once a job’s done. Exposure can bring on serious health problems—skin irritation, lung effects, heart issues. I’ve met chemists who tell stories about rashes or headaches after minor spills or careless handling. The risks sneak up, sometimes years after the work ends.
Throwing antimony potassium tartrate in the trash or pouring it down the drain is reckless. Heavy metals contaminate soil and water for decades. In my hometown, one creek still can’t support fish because of sloppy chemical dumping from the 1960s. Antimony compounds do not break down; small mistakes echo for generations.
Best practice starts with storage. Solid, leak-proof containers—often glass or specially lined plastic—keep spills contained. Labels should spell out the danger. In one university I visited, strong labeling and secondary containment stopped a near-disaster when a shelf collapsed. Instead of reaching groundwater, the mess got handled quickly and safely.
Antimony potassium tartrate usually counts as hazardous waste by EPA guidelines. That means professionals handle the disposal. Licensed hazardous waste collectors pick up materials, track everything in detailed logs, and use neutralization or stabilization processes. They might convert the compound to a less harmful form or pack it tight for secure landfills approved for toxins.
I’ve toured waste facilities where teams test chemicals for reactivity before disposition. Their training covers spill response, health protection, and long-term tracking. Nothing casual about the process. Every drum gets checked and logged, because missing a step puts real people and real places at risk.
Many labs don’t generate huge volumes of waste. Those folks sometimes partner with local hazardous waste collection events. My friend, a high school science teacher, stores her dangerous chemicals in a locked cabinet and makes a yearly trip to her city’s chemical roundup. She brings along the documentation and lets the professionals take over.
No protocol works if people don’t follow instructions. In places I’ve worked, regular safety training makes a difference. Staff learn the risks, review spill kits, and practice drills. New hires walk through the disposal process in person. Routine matters here; mistakes drop when people see safe disposal as normal daily business.
Tighter rules help, but the real gains show up when people see the value in safeguarding others and the environment. Investing in decent storage, arranging timely pickups, and keeping up with regulations go a long way. Better yet, look for alternatives to antimony potassium tartrate whenever possible. Substituting safer chemicals, updating equipment, or changing procedures can reduce risks up front. Less hazardous waste generated means fewer disposal headaches and a safer workplace.