Ammonium L-tartrate did not just appear in laboratory catalogs overnight. Its roots stretch back to early organic chemistry pioneers who explored tartaric acid’s salt derivatives in the 19th century. Research began with the investigation of tartrates as fermentation byproducts in winemaking. Chemists discovered that forming salts with various cations led to compounds with unique solubilities and crystallization properties. Ammonium L-tartrate surfaced both as a byproduct from extractions and as a compound deliberately synthesized to probe stereochemical questions—a pursuit that pushed the field toward the famous resolution of racemic mixtures. These discoveries helped shape the pharmaceutical and food industries’ approach to using and understanding chiral molecules.
Ammonium L-tartrate appears as a white, crystalline powder without much odor, though a faint tang can linger due to its tartaric acid content. It dissolves quickly in water and takes up space in multiple fields, from analytical chemistry to agriculture. The appeal often lies in its moderate cost and renewable starting materials. Manufacturers produce this salt in grades suited for different applications, including analytical, food, and technical specifications. Laboratories might request high-purity lots for chiral resolution or for standards in chromatography. Food and beverage processors add it to recipes or formulations for its acidity regulation or stabilization properties.
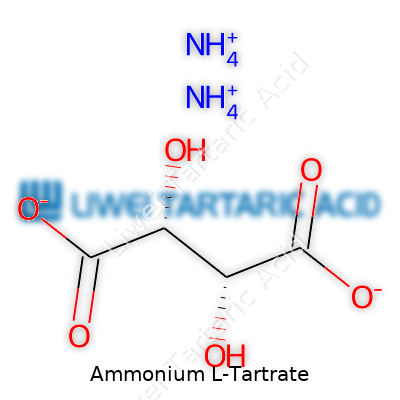
The salt carries the formula C4H9NO6 and a molar mass close to 167.12 g/mol. It forms colorless to white crystals, often monoclinic or orthorhombic, and easily dissolves in water but resists dissolving in most common organic solvents. Ammonium L-tartrate melts near 150°C, releasing ammonia and leaving an acidic syrup. With both carboxyl and ammonium groups present, this compound acts as a mild acid in solution, with pH readings between 3 and 4 at typical concentrations. The compound crystalizes with water of hydration if humidity allows, and dry forms may slowly absorb moisture in storage.
Suppliers list technical details that matter for the intended user. Purity levels exceed 99% for analytical use, and allowable heavy metal traces stay well below 10 ppm. Labeling should indicate batch number, expiration date, recommended storage (cool, dry place; away from acids or oxidizers), and health hazard warnings. Certificates of analysis frequently show test results for loss on drying, identification of L-tartrate by optical rotation or chiral HPLC, and ammonium ion content. Food-grade forms follow regional or international food additive codes, with regulatory compliance (FEMA/GRAS in North America, E number for Europe, generally E363).
The classic preparation starts with L-tartaric acid, derived from grape or tamarind sources, dissolved in water. Ammonium hydroxide gets added slowly, neutralizing the acid and producing a clear solution. Gentle evaporation or chilling prompts crystallization. Recrystallization from water, sometimes with a few drops of ethanol, improves clarity and purity. Tools remain simple: clean glassware, controlled heat sources, stirring rods. In industrial settings, the reaction takes place in stainless-steel tanks with careful temperature controls, followed by filtration to remove any insoluble matter and centrifugation to collect the crystals. Facilities handle byproducts with routine wastewater treatment protocols.
This salt acts as more than a simple buffer. Chemists value ammonium L-tartrate as a precursor or reactant in a handful of organic transformations, like resolving racemic mixtures of amino acids, alkaloids, or metal ions. In the lab, solutions of this compound interact with calcium or magnesium salts to form tartrate precipitates, which clean up hard water or assist in cation detections. Heating ammonium L-tartrate with oxidizers leads to the breakdown of organic fragments, making it useful for preparing certain oxalates or for reactions where a mild reducing agent is needed. It also plays a minor part in the synthesis of chiral catalysts, helping separate left-handed from right-handed molecules.
The product appears in catalogs as ammonium tartrate, ammonium L-tartrate, and monoammonium tartrate. Older chemistry texts sometimes refer to it as diammonium L-tartrate in error, though the mono salt is far more common. In pharmaceutical discussions, you’ll see the moniker “tartric acid, ammonium salt.” Inventory numbers may run under international CAS numbers or E numbers, and safety documentation uses standard hazard codes recognized globally.
Sticking with current best practices in laboratory and factory settings prevents most issues. The dust irritates eyes and respiratory passages if mishandled, so personnel work with local exhaust ventilation or wear dust masks. Storage cabinets marked for acids hold ammonium L-tartrate safely away from strong oxidizers or alkalis. Spills clean up easily with water, but the reduced environmental hazard does not mean users skip the safety goggles or gloves. Food-grade product acceptance depends on trace analyses for metals and organic impurities. Material Safety Data Sheets (SDS) detail emergency response for accidental ingestion, typically dilution and medical monitoring, since acute toxicity remains very low. Long-term, regular medical review of handlers helps avoid issues related to cumulative low-level exposure, though past regulatory reviews mark the compound as safe under normal use conditions.
Ammonium L-tartrate helps laboratories resolve chiral molecules, separating “left” from “right” forms with accuracy. Food scientists incorporate it as a tartness regulator for wines, candies, and jellies, where it lends a clean, tangy flavor. Producers in agriculture add it to nutrient or fertilizer blends for pH control, giving seedlings a gentle start in soils packed with minerals. Cleaning and metal plating businesses depend on ammonium tartrate’s ability to complex with metal ions, helping to keep processing baths stable and boosting plating quality. Teachers rely on this salt for crystal-growing kits used in basic chemistry education, thanks to its safety and predictable crystallization.
Chiral chemistry keeps uncovering new uses for tartaric acid derivatives. Labs aiming to develop greener synthesis methods investigate ammonium L-tartrate’s behavior as a mild acid or resolving agent. Researchers in analytical chemistry experiment with this compound as a buffer in electrophoresis and chromatography, probing how small tweaks in pH or concentration affect separation of complex mixtures. Agricultural technologists explore slow-release formulations, adding coatings or inorganic binders to the salt. Pharmaceutical groups have ongoing projects testing paired catalysts made from tartrate salts, trying to make production of enantiopure drugs cheaper and less wasteful. Through interdisciplinary collaboration, university groups and private companies share data on crystallization and stability, pushing practical applications forward.
Hundreds of animal studies over the years set a favorable toxicity profile for Ammonium L-tartrate. Oral LD50 values top 5000 mg/kg in rats, placing the risk on par with regular table salt. Inhalation or dermal exposures rarely cause anything beyond mild irritation, though direct contact with sensitive tissues needs quick rinsing. Chronic dosing studies in rodents show no significant buildup of adverse metabolites or organ damage. Regulatory panels in Europe, North America, and Asia review the salt’s use in food and pharmaceutical products every decade, re-confirming low acute and chronic risks, provided products are high purity and used as intended. Most stark warnings stem from risks due to low-grade material contaminated with heavy metals or improper storage conditions, which allow the breakdown to irritants or allergens.
Research into more efficient and sustainable production keeps picking up. Manufacturers scale up green chemistry routes, using bio-based tartaric acid feedstocks and closed reactor systems with energy-saving evaporation. Chiral synthesis—especially for new pharmaceutical compounds—looks set to depend even more deeply on tartaric acid derivatives like this one as the push continues toward cost-saving and minimizing waste in medical manufacturing. Next-generation food science, blending with probiotics or encapsulating active nutrients, considers ammonium L-tartrate’s gentle acidity and compatibility as both an additive and a stabilizer. In education, companies packaging science kits explore methods for colorful crystal growth or interactive experiments for classrooms. Toxicology research tracks cumulative exposures and focuses on identifying any breakdown metabolites for improved risk assessments. Production and supply chains shift toward greater transparency, reflecting consumer demand for clean labels and tight regulations across global markets.
Walk behind the scenes in a laboratory and you’ll spot Ammonium L-Tartrate on more than a few shelves. For those who haven’t handled this compound, it looks like a fine, white powder but holds a much bigger role than looks suggest. You’ll find it in chemical labs, food science, and even classrooms. It’s not some industrial oddity—plenty of teachers have set it out for hands-on crystal growth demonstrations that help spark curiosity in chemistry.
The full story of Ammonium L-Tartrate often starts in analytical chemistry. It’s a staple reagent for separating metals and purifying rare earth elements. The tartaric acid backbone attracts certain ions, allowing chemists to pull out specific metals from tricky mixtures. Researchers have found it helps pinpoint nickel, copper, and aluminum among a crowd of lookalike elements, which plays a big part in environmental testing—think drinking water or soil samples.
Ammonium L-Tartrate shows up in the food world, too. Quality control labs use it to test foods and beverages for contaminants or unwanted metals, keeping public health on track. Some food scientists even experiment with it in flavor stabilization research, thanks to its gentle reactivity and predictable structure, though it is not added directly to food as a common ingredient.
I’ve seen this compound make lessons lively in classrooms. Simple crystal growth kits let kids watch chemistry unfold in real-time, guiding them closer to science careers. Giving students hands-on experience moves chemistry out of textbook pages and into real life. There’s more energy in a class that mixes and measures, letting curiosity lead.
The United States Environmental Protection Agency (EPA) lists various uses and safety guidelines for ammonium tartrate, reflecting its value and potential risks if mishandled. Many university protocols treat it like any strong reagent: gloves, goggles, and careful handling. Research articles, such as those in “Analytica Chimica Acta,” show how it plays a part in separating metal ions more efficiently than older methods, helping industries meet tighter anti-pollution rules.
Like many chemicals, Ammonium L-Tartrate does present some hurdles. Disposal methods haven’t kept up with the scale at which labs produce waste. Some institutions have started pilot programs for reclaiming and reusing lab reagents, which could ease the environmental footprint. Larger manufacturers could back these practices by offering take-back initiatives or green chemistry solutions, following models already used for batteries and solvents.
The story of Ammonium L-Tartrate shows what happens when a tool blends reliability with adaptability. Broadening recycling efforts, improving educational outreach, and sticking to smart safety rules all keep risks low and rewards high. As part of a balanced chemistry lab, it makes scientific discovery a bit easier—one careful experiment at a time.
Picture a white, crystalline powder sitting in a lab. It goes by a formal name—Ammonium L-Tartrate—and sports a chemical formula: (NH4)2C4H4O6. Even if chemistry class wasn’t your favorite, this small compound packs practical value worth understanding, especially across food production, pharmaceuticals, and education. The lab-coated experts might talk about chirality or isomers, but at its core, ammonium L-tartrate shows how chemistry ties tightly into everyday life.
Ammonium L-tartrate combines two ammonium ions with the L-form of tartaric acid. Those four carbons and six oxygens sound dry on paper, but they reflect a natural origin, since tartaric acid comes from fruit like grapes or tamarind. I remember helping out in a small winery during grape harvest, and shoveling out spent fruit byproducts. Only later did I realize that those leftovers could feed the raw material stream for ingredients like this one.
Each part of the formula—NH4 from ammonia, C4H4O6 from tartaric acid—brings a role. The L- prefix tags the molecule as a specific mirror-image shape used by living things. It matters whether you’re using the L- or D- (right- or left-handed, so to speak) version. In food and pharma, only one side brings the right safety and fit for the body.
Bakers and soft drink makers run into ammonium L-tartrate as a leavening aid or acidity regulator. Anyone who has whipped up a meringue can appreciate how important the right balance of acidity feels—not just for taste but for structure. The FDA in the United States lists it as “generally recognized as safe” (GRAS).
In organic farming, this salt pops up in natural pesticide formulas. For students, it becomes a hands-on lesson in stereochemistry. I’ve watched a room full of teenagers light up when a molecule model snaps together, showing how these subtle shapes influence macro-level effects.
No one wants industrial chemicals mishandled, and ammonium L-tartrate isn’t innocent if tossed around carelessly. Though derived from natural sources, it can feed nutrient runoff if pitched into water streams. Responsible disposal and following material safety data sheets mean more than red tape—they keep real damage at bay. The European Food Safety Authority and other regulators update standards and limits in response to new science.
In my experience, people care most about trust—with their food and with the companies using these ingredients. The only real way to build that trust involves clear labels, third-party testing, and plain-language explanations. Every batch starts with chemistry, but it ends with real people reading the label or using it in a recipe. Understanding the story behind (NH4)2C4H4O6 connects dots between science, health, and the daily decisions we all make. That’s how chemistry keeps a place at the kitchen table, not just in the textbook.
Ammonium L-tartrate doesn’t often end up in kitchen cupboards, though it pops up sometimes in food technology discussions. It’s a salt made from tartaric acid, which comes from fruits like grapes, mixed with ammonia. Anyone who grew up around vineyards or spent time in a winery probably remembers tartaric acid in some form – it’s what causes those crystal-like deposits in wine bottles. L-tartrate’s cousin, potassium bitartrate, is much better known; folks call it cream of tartar and use it for baking.
Plenty of substances get classified as safe just because they’re in familiar food, but it’s best to check specifically about ammonium L-tartrate. According to the US Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA), ammonium tartrate falls into the “generally recognized as safe” (GRAS) category when added to food in small amounts. The GRAS tag means those food safety organizations have enough data showing no known adverse effects for the quantities typically used.
Most food-grade ingredients undergo toxicology studies that track what happens at various doses in both animals and people. Scientists have found that tartrates are well-tolerated at levels used for food processing. High doses, on the other hand, can bring on digestive issues like stomach cramps, nausea, or diarrhea. No one is asking people to sprinkle ammonium L-tartrate on their toast, and no respected recipe calls for anything close to a dangerous amount.
Mostly, manufacturers use ammonium L-tartrate as a food additive for specific effects like acidity regulation. It fits best in products where the goal is to tweak tartness or help baked goods rise evenly. In winemaking and brewing circles, it can sometimes play a role in improving the taste or stability of a finished product. Anyone who’s made sourdough or bread knows how acids affect flavor and texture.
Its chemical cousins are found naturally in food, so our bodies are wired to process modest amounts. People who have eaten fruit, had a glass of wine, or enjoyed certain candies already met tartrates. The ammonium side just means it partners with a different mineral. Chemical safety depends on both dosage and frequency, not just the scary sound of the name.
One problem with food additives, in general, involves public trust. Folks get nervous if they don’t recognize the ingredient or feel out of the loop on what goes into food. There’s a lesson for scientists and food makers here: everyone feels better when ingredients and their limits are spelled out clearly. Labels that use plain language and regulators who keep up public communication would make a big difference.
People with kidney trouble or certain metabolic disorders ought to consult a doctor about what food additives to avoid, just to be safe. For the rest of us, overloading on any additive rarely makes sense. Balanced diets, with plenty of whole foods, put worries like this far in the background.
Researchers continue tracking the long-term impacts of all food additives, and new data appears all the time. Food safety changes as scientists review new samples, run fresh studies, and sift through health reports. Sticking with regulatory guidelines for additives like ammonium L-tartrate keeps food both innovative and trustworthy.
Ammonium L-Tartrate pops up on lab shelves for a few good reasons. It’s useful in chemistry and biology work, and some folks rely on it for more specialized research. The way people treat chemicals between those busy experiments gets overlooked. Taking the time to handle storage properly really means protecting more than a product: it’s about safety, reputation, and sometimes even the next discovery down the line.
This salt draws moisture straight out of the air. Leave a bottle of it open for a few hours, and there goes its dry, powdery texture. Lumps form, and nobody wants to scrape a sticky mess for an accurate measurement. That’s why folks working in research or industry keep the container closed every single time, opening it just long enough to scoop what they need. Old timers in the lab know that airtight jars—think glass with tight screw tops or those classic plastic containers with strong seals—mean fewer mistakes. On muggy days especially, storing Ammonium L-Tartrate in a desiccator works wonders. Silica gel packets tucked in with containers help by keeping things drier, too.
I learned a hard lesson during my early lab years: leaving chemicals beside the window, even for a couple days, changes them. Sunlight heats up containers and can trigger subtle reactions. Ammonium L-Tartrate prefers a cool, dark corner—inside a cabinet, away from radiators or sunlight. Heat speeds up the breakdown of many compounds and no one wants unexpected results in the next experiment. Some companies back up this advice by giving storage temperature ranges right on the label. If the instruction says “store at 15-25 °C,” check that the spot you pick actually keeps within those numbers, not just most of the year, but during a random heatwave, too.
It shocks me how often folks set chemicals next to their snacks. Cross-contamination risks never get less serious. Ammonium L-Tartrate doesn’t belong in the kitchen or fridge, unless that fridge carries only research samples—never family leftovers. Good labeling makes a world of difference. Mark the jar well, use hazard stickers, and write the purchase or open date. These habits help track age, so no one uses expired materials. Separating chemicals by class—keeping organics with organics, oxidizers well away—protects against accidental mix-ups that can get dangerous fast.
Accidents aren’t rare. Maybe a lid didn’t close right, or a jar tipped over. Knowing how to handle cleanups makes a difference. Scoop up the powder, avoid breathing dust, and always use gloves. Toss it in a properly labeled waste container; don’t treat it like regular trash. If the material’s old and the label’s faded, it’s best to treat it as hazardous waste, not worth the health gamble. Safety Data Sheets (SDS) spell out these protocols. It pays to print a copy, stick it near your bench, and actually read it before trouble comes knocking.
Getting storage right isn’t about being perfect; it’s the consistent habits that add up. Open the jar briefly, seal it tight, put it in the right spot, and keep it dry. Safety and care show respect for every colleague who’ll reach for that jar after you. A tidy, well-organized chemical shelf means more reliable results and fewer headaches for everyone down the line.
Ammonium L-Tartrate doesn’t make regular headlines but those who have spent time in a university chemistry lab have most likely come across it. The solid, white and slightly crystalline compound has earned a steady spot on chemical shelves for good reason. Its formula might sound intimidating—C4H11NO6—but its uses and importance are much more approachable. Even outside of textbook chemistry classes, it crops up in food technology, fermentation research, and even environmental analysis.
If you scoop some of this compound from a chemical bottle, you’ll notice that it forms as small crystals—sometimes needles, sometimes tight clusters. No fancy sparkle, but the powder throws off a ghostly white shade and doesn’t attract much attention unless you know what to look for. There’s no scent, so don’t expect a sharp tang or warning whiff. It doesn’t clump in normal lab air, which I can attest makes for easier handling and weighing on sensitive laboratory balances. In my own work, a jar can last for months without much change in texture, which points to a solid shelf-stability.
Pick up a beaker of water and add a small spoonful of Ammonium L-Tartrate. Pretty soon, it vanishes—dissolving easily and completely in cold water. This solubility stands out, especially for someone like me who values low-fuss chemicals. No special equipment or prolonged stirring, just an even, clear solution every time. Many organic salts can leave a cloudy residue or take forever to go into solution, but you don’t run into that issue here. If you scatter it on a humid day, it doesn’t noticeably draw moisture from the air, avoiding the sticky mess hygroscopic substances cause.
Heat up Ammonium L-Tartrate, and it shows the profile of a typical organic salt. It holds together below 170 °C, but push it hotter and the substance starts to break down, giving off ammonia and that unmistakable tang of organic decomposition. In routine school labs, this heat sensitivity rarely causes trouble, because experiments usually keep to mild water-bath temperatures. From experience, overheating this compound makes for tough cleanup and an unwanted smell. Using it within a controlled temperature range helps preserve its properties and protect sensitive equipment.
Properties like water solubility, appearance, and heat response shape what scientists and industry folks can do with this salt. In the food business and brewing, predictability and easy dissolving keep processes running smoothly. Schools and research institutes favor it because students learn about organic salt behavior without dealing with fumes or tricky cleanup. Waste management becomes much simpler with a product that dissolves fully and leaves little residue.
For those looking to find alternatives to more hazardous chemicals, Ammonium L-Tartrate marks an example of a safer, more manageable choice. As tighter safety and sustainability rules roll out—especially in schools and small-scale labs—compounds with these straightforward physical traits are set to become even more relevant. Exploring alternatives and educating the next generation about responsible chemical choices can help keep safety and curiosity balanced in the lab.