The road to understanding and utilizing 3-Mercapto-1,2-propanediol, often known as thioglycerol, stretches back to the early boom years of organic chemistry in the 19th century. Chemists first isolated it while experimenting with compounds containing sulfur and oxygen, two elements that play crucial roles in metabolic and material science. Once researchers mapped its molecular formula, C3H8O2S, it didn’t take long to find key differences from similar diols like glycerol, especially the reactivity added by the mercapto (thiol) group. Observing its quick reactions in basic laboratory settings, early industrial chemists realized 3-Mercapto-1,2-propanediol’s unique redox and binding properties. Over time, this compound found steady footing among both academic researchers and process engineers who recognized its potential for synthesis, catalysis, and, eventually, pharmaceutical and cosmetic applications.
3-Mercapto-1,2-propanediol isn’t just another chemical on the long shelf—its combination of alcohol and thiol groups unlocks a versatility that few small molecules can claim. This compound often comes as a clear to pale yellow liquid, carrying that tell-tale sulfurous odor. Regular users, from synthetic chemists to industrial operators, value it for strong nucleophilicity and high solubility in water and polar solvents. It merges two reactive functionalities on a tight three-carbon backbone, setting up a variety of modifications for broader end-use, especially those that demand a sharp nucleophile or reducing agent. Whether as a stabilizer in certain formulas, a chelating agent in metallic processing, or a building block for more active molecules, its role grows as demands for specificity in chemistry rise.
Solid data anchors the widespread use of 3-Mercapto-1,2-propanediol. This compound’s molecular weight stands at 108.16 g/mol, with a melting point around 40°C and a boiling point near 210°C at atmospheric pressure. Its density usually falls between 1.3-1.4 g/cm³ at room temperature. Its high polarity and hydrogen bonding are mostly due to those two hydroxyl groups, helping the molecule dissolve in water and most alcohols. The sulfur atom, sitting as a thiol group, sets the stage for redox activity and gives chemists a handle on further transformation. This molecule reacts rapidly with electrophiles and promotes disulfide formation, especially under oxidative conditions, which can sometimes complicate storage and handling. Air and light exposure demand extra care, particularly since the compound can readily oxidize, forming disulfides that may change its effect in a formulation or process.
Quality and signaling matter a great deal in chemical procurement. The best suppliers offer 3-Mercapto-1,2-propanediol at varying purities, with technical and analytical grades available. Standard labeling provides the CAS number (96-27-5), UN number, structural formula, and key hazard statements per the GHS system, flagging its irritant properties and need for controlled storage. Users pay close attention to expiry dates, recommended storage temperatures (typically below 25°C), and the lot-specific assay to safeguard repeatable performance. The top end of the market expects robust quality assurance: impurity profiles, moisture content by Karl Fischer titration, and metal analysis help guarantee that the chemical won’t cause downstream issues, especially in pharma or electronics processes where contamination spells disaster.
Most 3-Mercapto-1,2-propanediol in the marketplace comes from chemical synthesis, where the typical route begins with the reaction of epichlorohydrin and hydrogen sulfide, followed by hydrolysis. The process starts in a controlled reactor, where epichlorohydrin, a readily available industrial chemical, reacts under moderate temperatures with hydrogen sulfide in the presence of a catalyst. This step replaces the chlorine atom with a thiol group, creating a key intermediate that undergoes subsequent hydrolysis to open the epoxide ring and produce the diol. Careful distillation at reduced pressure ensures high yields and product stability. Modern improvements focus on reducing byproducts and energy consumption, nudging process chemists toward greener, safer approaches, such as employing non-chlorinated precursors or adopting catalytic systems that lower waste. Researchers continue to scale up alternative methods that cut environmental impact while improving selectivity, an ever-important pursuit as regulatory and sustainability demands increase.
Few small molecules rival the reactivity portfolio of 3-Mercapto-1,2-propanediol. In research labs across the globe, scientists exploit its bifunctional nature to anchor new chemical frameworks. The thiol group, prized for its nucleophilicity, reacts cleanly in Michael additions, alkylations, and conjugations with iodoacetates, making it indispensable for modifying biomolecules and polymers. The diol segment participates readily in esterifications or etherifications, broadening the scope for introducing protective groups or linking to larger molecules. In oxidative conditions, the thiol oxidizes to disulfide, allowing self-assembly and cross-linking applications. This suite of chemical transformations underlies many uses in drug development, biochemical assays, and material science, giving researchers robust control over molecular architecture and function. Synthetic chemists learn to mind its sensitivity to air and light, which can shift both the speed and outcome of critical reactions unless kept under inert atmosphere or with antioxidants.
Different industries and countries know this compound by several names, each hinting at its roots or dominant features. Among the common synonyms are thioglycerol, 1,2-dihydroxy-3-mercaptopropane, mercaptoglycerol, and β-mercapto-1,2-propanediol. In commerce, product catalogs might list it under TG, a simple shorthand for repeat buyers. The naming diversity sometimes trips up new users hunting through resource catalogs, so keeping a clear record of synonyms avoids procurement mistakes or regulatory confusion. Regulatory bodies, such as the FDA and EFSA, reference the CAS number to cut through naming differences, making compliance and labeling checks more straightforward across international borders.
Handling 3-Mercapto-1,2-propanediol calls for solid operational discipline. Skin and eye contact can trigger irritation, so users rely on gloves, goggles, and good ventilation. Its strong odor signals the potential for local air discomfort, particularly in poorly ventilated labs or shops. Safety data sheets recommend working with it in fume hoods, storing it in tightly sealed amber bottles to guard against oxidation, and segregating it from oxidizing agents. The chemical’s moderate toxicity keeps it out of open-air reactions at scale, and proper spill protocols matter: silica, vermiculite, and rapid containment reduce risk if a leak occurs. Regulations in industries like pharmaceuticals and food demand rigorous process validation, documented cleaning procedures, and strict batch tracking to trace any exposure or contamination incident. By following these standards, users reduce incidents and keep teams safe, preserving health, productivity, and regulatory standing.
3-Mercapto-1,2-propanediol weaves its way into a surprising range of industries. It stabilizes enzyme solutions in biotechnology runs, where the thiol group helps maintain protein structure by reducing unwanted disulfide bonds. In cosmetic formulas, it acts as a reducing agent for hair reshaping products, such as permanent waving solutions, taming the natural structure of keratin by snipping disulfide linkages and allowing new shapes to set. Electronics manufacturers use it as a chelating and cleaning agent, with the sulfur atom binding to trace metals and cleaning up circuit paths during critical production steps. Analytical chemists appreciate it as a sample stabilizer, adding it to reagents that need protection from oxidation or metal contamination. The pharmaceutical sector tests its derivatives for antimicrobial effects and as scaffolds in drug candidate designs. Throughout, the compound’s reactivity and dual functionalization give users a strong toolkit for applications demanding high specificity or robust control over molecular transformations.
Academic and industrial R&D teams keep finding new ground for 3-Mercapto-1,2-propanediol, digging into both its chemical utility and performance in emerging applications. For decades, university labs have pushed its use as a tether in protein labeling experiments, where the diol ensures water solubility and the thiol anchors to reactive groups in proteins or probes. Material scientists mix it with polymers and nanoparticles, tuning their mechanical and electrical properties by taking advantage of the thiol’s strong bonding. Ongoing work explores its use in hydrogels that respond to redox changes, which could enable next-generation medical devices or responsive wound-care products. On the manufacturing side, green chemistry researchers experiment with bio-derived starting materials, hoping to cut carbon footprints and reduce potentially hazardous byproducts often associated with current synthetic processes. Patents and publications keep rising, pointing to continued interest and growing opportunity for more efficient and targeted uses.
The safety of employees and end-users depends on clear-headed toxicity research, which covers both acute and chronic exposure risks through all possible contact routes. Animal studies show moderate toxicity from ingestion and skin contact, sparking regulations that set strict exposure limits in occupational settings. Inhalation at high concentrations triggers nausea and respiratory irritation, while prolonged skin exposure in clinical settings can lead to dermatitis. Environmental toxicity profiles remain of interest as more regulators watch the fate of organosulfur compounds in wastewater and soils. Researchers investigate breakdown pathways, showing the diol portion rapidly biodegrades while the thiol hangs on longer especially under low-oxygen conditions. Routine lab surveillance monitors surface and air concentrations, ensuring compliance with occupational safety benchmarks and reducing long-term exposure risk to workers and communities alike.
The horizon looks wide for 3-Mercapto-1,2-propanediol. Performance-driven sectors, from electronics to personal care, demand chemicals with highly specific and predictable action. Early success with protein modification and material binding has chemists looking for more ways to incorporate thioglycerol analogues into drug delivery, disease diagnostics, and even next-generation smart surfaces. Organic synthesis continues shifting toward efficiency and sustainability, so improved routes that lower hazardous effluents or tap renewable feedstocks can only push adoption further. Sustainability in packaging and supply chains grows as a selling point, rewarding those producers who invest in greener methods and transparent documentation. As regulatory science moves to capture ever finer details of chemical fate and toxicity, robust research into environmental impact and safer derivatives will continue to pay off. For students and professionals alike, familiarity with the practical uses, risks, and benefits wrapped up in 3-Mercapto-1,2-propanediol remains a valuable asset for innovation and responsible chemical stewardship.
Step into any facility mixing up products for cosmetics, pharmaceuticals, or coatings, and you'll spot bottles and drums marked with unfamiliar names. One such label is 3-Mercapto-1,2-Propanediol. Folks in specialized labs or on factory lines know it for its reactive power, but most people walk right past without giving it a thought.
In the world of chemistry, 3-Mercapto-1,2-Propanediol isn’t flashy, yet it gets results. Take the paint on your walls. This compound often turns up in water-based paints and coatings. Its main job: prevent corrosion. It binds strongly with metal ions and disrupts the little reactions that cause rust. This property cuts down on the need for heavy metals in paints, which means safer walls for homes, schools, and offices.
Swing over to cosmetics and personal care. Ingredients lists can read like alphabet soup, but many curl creams or hair treatments include 3-Mercapto-1,2-Propanediol. Chemists value it for its ability to manipulate hair’s structure. That signature “perm” smell? Part of it traces back to this compound. It keeps cysteine bridges in hair manageable and makes long-lasting styles possible.
Medicine makers use 3-Mercapto-1,2-Propanediol less for appearance and more for chemical guts. It serves as a reducing agent. This means it helps trigger, speed up, or stop certain reactions. Manufacturers lean on it when crafting drugs where sulfur groups need tweaking or protecting. Its toxicological profile demands careful handling. Overexposure, particularly in closed spaces or through skin contact, creates real risks.
Health and safety outfits, including regulatory teams from Europe to North America, stress the importance of protective gear in workplaces using this substance. Regular air monitoring, gloves, and ventilation aren’t optional. Mistakes with safety don't just cost money. They can put workers at real risk—stories make the rounds every few years of folks winding up at the clinic after careless mishandling.
Chemicals like 3-Mercapto-1,2-Propanediol wash down drains, find their way into water systems, and enter the wider world. Once out in the open, they can stress aquatic life. Fish, for instance, respond poorly to exposure over time. That means responsible disposal needs attention at every level.
Science never sits still, and there’s real energy behind figuring out alternatives that carry less risk for people or landfills. Some manufacturers now hunt for greener synthesis methods, hoping to keep performance high without spreading toxic leftovers.
Anyone working with this compound owes it to themselves and their community to understand the risks, follow the best available science, and improve systems where possible. Training, strict adherence to process, and open communication with environmental regulators can make a difference. Whenever new data about toxicity or degradation crops up, responsible actors put it to work in updated protocols.
At the end of the day, a chemical like 3-Mercapto-1,2-Propanediol holds up a mirror to the broader challenge facing science and industry: using strong tools without letting short-term gains overshadow the long-term health of people and planet.
3-Mercapto-1,2-propanediol, also called thioglycerol, features in industries like photography, pharmaceuticals, and specialty chemicals. It’s not on the average person’s radar, but in many labs and factories, workers handle this substance every day. Just because it doesn’t spark much buzz outside technical circles doesn’t mean people should ignore its risks. Like many thiol-containing compounds, it has a reputation for a strong, unpleasant odor, which usually serves as a red flag in chemical safety conversations.
This chemical packs a punch if mishandled. Its toxicity comes from its ability to interact with proteins and enzymes, which has real-world implications, particularly when it touches skin or gets inhaled. Direct contact causes irritation—redness, itching, or burning are common. Its vapors, especially in closed spaces, can lead to headaches, dizziness, and nausea for those caught off guard by a leaking bottle or a splash during mixing.
Those familiar with safety data sheets know 3-mercapto-1,2-propanediol isn’t a casual workplace risk. According to the National Institute for Occupational Safety and Health, this chemical acts as a skin and respiratory irritant, so workers need more than a pair of gloves and hope on their side. Prolonged exposure, especially without proper ventilation, raises chances of more severe symptoms with chronic irritation and possible allergic responses building up over time.
Hazardous chemicals don’t just affect those at the lab bench. If spilled into the environment, 3-mercapto-1,2-propanediol poses risks for aquatic life since thiols in waterways can disrupt natural processes in fish and plants. Regulatory agencies place strict guidelines on storage and disposal, but history shows rules alone never stop every spill. I’ve worked in labs where tired or rushed staff cut corners, only to realize too late that a chemical like this had leaked, forcing days of clean-up and paperwork.
Certain communities living near industrial facilities shoulder a heavier burden, too. Runoff incidents can cause foul odors and potential water contamination, fueling distrust between factories and neighbors. People in these situations often wonder whether companies do enough to prevent harm. Real transparency and strong enforcement help, but so does community science where locals test air or water and demand answers.
Staying safe with 3-mercapto-1,2-propanediol takes good habits more than technical heroics. Ventilated workspaces and reliable containment systems stop small mistakes from growing into full-blown emergencies. I’ve seen the difference regular safety drills make—when everyone knows their role, response times drop and accidents stay small. Training staff to recognize the warning signs of exposure, like skin irritation or headaches, brings attention to problems before they escalate.
Research and development teams can step up by tweaking chemical formulations, finding safer substitutes wherever possible. Investing in real-time exposure monitoring helps catch leaks and spills early. On a broader scale, stricter oversight ensures companies don’t treat regulations as paperwork, but as a baseline to beat. Communicating hazards openly with workers and nearby communities goes a long way to building trust and keeping everyone healthy.
Personal vigilance matters most. No label or safety protocol replaces taking responsibility for what chemicals can do. Stay informed, voice concerns, and respect the risks—these steps turn a hazardous material into just another tool, handled with respect and care.
3-Mercapto-1,2-propanediol, often labeled as thioglycerol, acts as a reducing agent and stabilizer in several industrial and lab processes. Only a few people outside the lab encounter it, but for chemists and those in related trades, storing this compound the right way genuinely matters. Safety is at stake, plus the compound’s effectiveness sinks if ignored.
Anyone handling thioglycerol spots its strong, lingering odor quickly. Sulfur compounds never go unnoticed. A leaky or loose container soon creates a very unpleasant workspace. Worse, the product attracts water from the air. Over time, humidity leads to decomposition, releases more foul-smelling gases and ruins the original purity. Leaving this stuff open on your bench or shelf, contamination will creep in and soon, that detail sneaks its way into your results.
I've worked with substances that love moisture and light, and once you lose a batch to air or sun, you don't take storage conditions for granted. 3-mercapto-1,2-propanediol is no different. The bottles often arrive sealed tight with a screw cap and usually have a layer of nitrogen or another inert gas trapped above the liquid inside. This gas blanket fends off oxidation, and stops the product from breaking down. Keep the original bottle unless you have access to equally tough packaging.
A fridge or a dedicated chemical cold room works best. High temperatures speed up chemical breakdown. Once, someone stored this compound in a regular supply closet near a steam pipe, and by the time we checked on it, crystals had formed around the cap and the contents smelled worse than rotten eggs. Cooler temperatures slow these changes way down and let you use the product until the labeled expiry.
Moisture control matters even more. I’ve seen bottles gather condensation after being opened on a humid day. Each time the cap comes off, a little air sneaks in, and after a while, you’ll notice color changes or weird precipitates. Always use dry gloves or tools, and seal the bottle immediately.
Also, strong light, especially sunlight, can trigger unwanted chemical reactions. I store light-sensitive items in amber glass or well-closed plastic bottles, tucked away from windows or strong LED bulbs.
It’s easy to mix up similar-looking chemicals, especially if you keep your shelves crowded. Double-check the label for hazards and use a bright sticker if it needs special handling. From my own lab days, I write the opening date on every bottle. That helps spot the old stuff lurking in the back before it causes problems.
If you spill 3-mercapto-1,2-propanediol, the smell will get attention fast, but irritations to skin and lungs follow. Store it within easy reach of gloves, goggles, and a spill kit. Waste solvent containers must stay closed tight since fumes affect both safety and air quality in tight spaces.
While regulations and data safety sheets offer foundational guidance, personal routines round out true safety. Share what works, keep bottles sealed tight, and teach each other what to watch for. Applications of 3-mercapto-1,2-propanediol run smoother when storage habits reduce exposure risks and preserve its chemical punch.
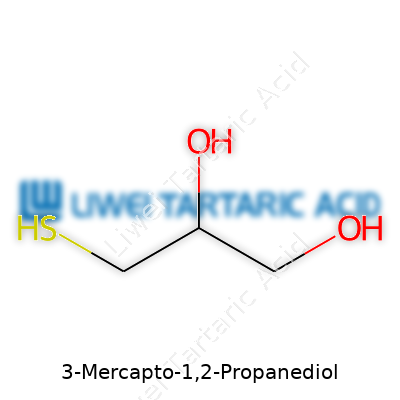
3-Mercapto-1,2-propanediol, better known to some in the laboratory as thioglycerol, brings something interesting to the table. The chemical structure looks simple but packs a lot of significance into just a few atoms. Picture a three-carbon backbone (propane), two alcohol groups, and a mercapto (thiol) group. The proper layout: HS–CH2–CHOH–CH2OH. Two hydroxyl groups and a thiol all hanging off neighboring carbons.
Each group serves its own purpose. The two –OH groups make this molecule a diol, a familiar class that shows up everywhere, from antifreeze to cosmetic humectants. The thiol group (–SH) holds extra weight in chemical reactivity, often acting as a nucleophile and reducing agent. Its smell won’t win any awards—it gives off that classic sulfur whiff most folks would recognize from things like garlic and skunk spray, a feature that shouldn’t be underestimated in handling.
Saying a compound has –SH and –OH groups on adjacent carbons might seem like trivia. Spend an afternoon working with proteins in the lab, and the importance becomes clear. Thiols break disulfide bonds with ease. This makes 3-mercapto-1,2-propanediol useful for denaturing or reducing proteins, especially where scientists want to unravel structure without much fuss. The molecule’s water solubility (thanks to two hydroxyls) keeps experiments smooth, blending easily into aqueous buffers.
The structure isn’t only about what it can break. In organic synthesis, the thiol group lets chemists play around with new bonds. Sometimes, it acts as a linker or starter for more elaborate molecules. It also crops up in cosmetics and lotions. The hydroxyl groups act as humectants, pulling moisture into skin. Add the sulfur end, and now you’ve got a compound that stabilizes or modifies other ingredients.
Hearing about chemical structures can read like a dry recitation, though the real story is how these choices affect people’s work and well-being. Compounds with thiol groups deserve some respect. A little on your gloves, and the smell might linger through the rest of the day. I once made the mistake of using too loose a stopper—by the time I noticed, everyone knew who’d been working with thioglycerol. Safety goggles and solid ventilation become non-negotiables.
Exposure to thiols can irritate skin and mucous membranes. Flushing with copious water works; using a chemical fume hood turns an unpleasant ordeal into a routine step. Organizations like OSHA advise routine controls and proper training before handing anyone a bottle of this stuff. In an age so focused on both lab and consumer safety, relying on the structure–function relationship gives a starting point for understanding risk.
The path between “here’s a neat molecule” and “safe, effective use” isn’t always straight. Good product design relies on understanding structure—and on actual human experience. I learned to appreciate the quirks of 3-mercapto-1,2-propanediol not from textbooks, but through one too many glove changes and trouble-shooting in a cold room. Chemical structure shapes real decisions: how we store, mix, neutralize, dispose.
Factoring in both reactivity and health impacts, teams can shape better products and procedures. Clear labeling, training, monitoring air quality, and updating protocols make all the difference. Chemicals don’t become less dangerous by being common. Reliable supply and transparent manufacturer practices go a long way in keeping both bench scientists and end users out of harm’s way.
Anyone working around chemistry labs or the pharmaceutical industry might bump into chemical names that seem to have been designed to confuse anyone sane. 3-Mercapto-1,2-propanediol fits right into that category. I learned early in my lab days that this mouthful goes by simpler names, used far more often in real conversations, textbooks, and bottles on the shelf. The most common is Thioglycerol. Some textbooks and suppliers call it β-Mercapto-1,2-propanediol, dropping numbers and rearranging syllables, but most people stick to thioglycerol. Once in a while, you’ll see glycerol thiol or even just β-mercaptopropanediol, though those versions pop up less often outside specialized literature.
Using several names for one chemical isn't just confusing—it's risky. I once watched a colleague nearly mess up a buffer solution because she didn't realize a bottle marked "thioglycerol" was what our protocol called "3-mercapto-1,2-propanediol." Inaccurate labeling can lead to wasted time or ruined experiments, but in some cases, exposure to the wrong substance brings real health hazards. Synonym confusion means a chemist could grab the wrong reagent, or a safety officer might overlook a dangerous compound.
The bigger chemical suppliers have tried to standardize labels using CAS Numbers. 3-Mercapto-1,2-propanediol sits at 96-27-5. Reliable suppliers list all common synonyms along with the number, but university storerooms and old textbooks still carry the legacy of multiple names. So that raises the stakes for anyone working with these chemicals, from researchers to students.
I once took part in a lab safety workshop where a group of postdocs debated the safety profile of “thioglycerol” and “3-mercapto-1,2-propanediol,” not realizing they were talking about the same substance. This wasn’t some rookie mistake—the confusion came from years of using names from different research papers and suppliers. The main hazard with this chemical is its potent smell (think rotten eggs), and it has a knack for irritating skin and eyes. MSDSs (Material Safety Data Sheets) will list all its aliases, but not everyone reads those closely enough.
Open communication lowers risk. Experienced lab managers urge newcomers to double-check both the CAS number and the list of synonyms before starting a procedure. Pharmaceutical manufacturing plants and quality control labs train technicians to search for hazards under every alternate synonym. It helps to run inventories with a synonym list by your side, so you don’t overlook stock that’s hiding under a different label. These steps make a difference in workplaces where a simple naming confusion can interrupt workflows or threaten safety.
Digitization helps. The best online chemical databases now attach a long list of synonyms, CAS numbers, safety data, and common uses to every substance profile. Supplier transparency can go further—more labels on containers should print every common name alongside the main one, especially for anything with a strong odor or health risk. Training programs include units on chemical synonyms and labeling, so nobody new in the lab feels like they’re learning a secret language. It's a small step, but one that builds a safer and more productive environment for everyone working with chemicals like 3-mercapto-1,2-propanediol, whatever you call it.