Chemistry offers countless discoveries that shift the ground beneath many fields. 3-Amino-1,2-propanediol grew out of early experiments in amino alcohol synthesis. Researchers in the early twentieth century hunted for molecules blending water solubility with reactivity. By stitching an amino group onto propanediol, scientists opened up a new group of intermediates. At first, the focus stayed narrow. Lab teams were drawn in by how this molecule could act as a chiral building block. Pharmacology and agrochemical innovators saw potential, leading to the molecule’s rise in the synthetic toolkit. Early manufacturing still faced hurdles following the first literature descriptions, with chemists contending with mixed yields and inconsistent purity, but determination to solve these problems set a path that brought greater control and standardization across the globe by the 1970s.
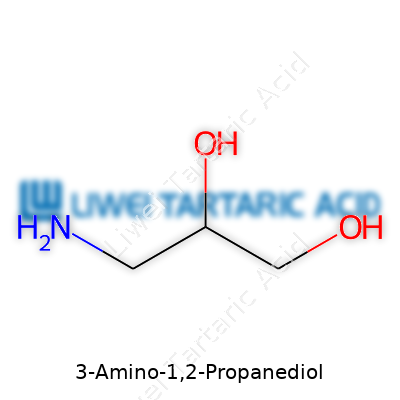
3-Amino-1,2-propanediol, known in the field as serinol, stands out for blending two alcohol groups and one amine into a three-carbon backbone. In pure form, it usually comes as colorless crystal or viscous liquid, and its dual functional groups offer a handle for all kinds of follow-on modifications. Chemical vendors sell it in various purities, tailored for specialty manufacturing and research grades. This molecule’s unique reactivity explains its range, making it essential not just for chemical synthesis but also as an analytics reference standard and component in specialty formulations.
In the lab, serinol shows up as a non-volatile, water-loving solid with a melting point near 50°C and a boiling point rising past 200°C. Its high water solubility reflects all those hydrogen bond donors and acceptors jammed into a small space. It dissolves instantly into polar solvents—water, methanol, and ethanol—though it shies away from hydrocarbons. The molecule sits somewhere between basic and neutral, thanks to the primary amine. Users in synthesis rely on these properties to predict reactivity and ensure safe handling. The strong hydrophilicity makes this compound popular in water-based reactions, and those alcohol groups give plenty of room for new modifications from simple esters to more complex derivatives.
Manufacturers provide serinol with strict technical data: purity stated as a percentage (ranging from 95% up to 99% for research), measured water content, and detailed impurity profiles. Vendors label all primary hazards, CAS number, lot number, and recommended storage instructions. Labels usually warn about eye and skin irritation risks. In some countries, transportation falls under the scope of regulations for amino alcohols, including packaging instructions to prevent leakage or contamination, plus batch identification to support traceability for regulatory audits.
Chemists typically prepare 3-amino-1,2-propanediol starting with readily available precursors like dihydroxyacetone or glycidol. One common route uses ammonia addition to glycidol, harnessing controlled temperature and pH to steer the reaction and reduce side products. Afterward, purification steps—distillation or recrystallization—strip out unwanted byproducts. This stepwise approach means anyone in a production-scale facility needs careful monitoring to handle exothermic points and keep yields high. Smaller labs may use a similar workflow, though they often work with finer batch control. Clean water is a must to ensure quality, and analytical checks for residual ammonia or byproducts keep everything up to pharmaceutical or analytic grade standards. Improvements in process controls over decades have pushed yields higher and cut down on waste.
The versatility of serinol stems from its dual functionality. The primary amine can react with acids to form amides, or with aldehydes and ketones to form imines. The alcohol groups allow etherification, acylation, and oxidation. Research teams frequently modify serinol to serve as a backbone in specialty ligands for catalysis, or as an intermediate in chiral auxiliary synthesis. Pharmaceutical makers value it in building beta-blockers, antiviral agents, and antibiotics. Radiolabeling the compound opens it up for tracer studies, and its reactivity helps form useful downstream derivatives for everything from surfactants to biocompatible polymers.
In industry and academia, 3-amino-1,2-propanediol appears under several alternative names. Chemists call it serinol, referencing its link to serine. Some suppliers list it as 1,2-dihydroxy-3-aminopropane or 2-amino-1,3-propanediol. National and global chemical catalogs cite unique synonyms, ensuring it is recognizable in almost any lab. The standardized CAS identifier makes supply chain tracking easier, and crossing language barriers does not hide its identity for regulatory reviews.
Handling serinol takes care. Its amine group can irritate eyes, skin, and respiratory tissue. Standard lab gloves, goggles, and fume hoods are required for scale-up work and during transfers. Users in chemical plants follow site-specific protocols involving emergency eyewashing and spill cleanup plans. Storage away from strong oxidizers and acids ensures a long shelf life and reduces incident risks. Waste disposal methods turn to aqueous neutralization and collection as mandated by local and national regulations. Experience shows that rigorous adherence to MSDS sheets and chemical hygiene plans keeps incidents low, and proper documentation at each step means operators can respond fast if issues emerge.
Few chemistries span as many industries as 3-amino-1,2-propanediol. In pharmaceuticals, its chirality enables the design of bioactive molecules where traditional amino alcohols fall short. It’s a staple in beta-blocker drug manufacture and helps scientists build antibacterial precursors. Polymer scientists add it to specialty polyesters and polyurethanes to introduce flexibility and hydrophilicity. Agrochemical developers produce novel herbicides and growth regulators based on its reactive skeleton. In analytical chemistry, it finds use as a derivatizing agent. Research labs test new radiolabeled versions for metabolic tracing, and formulation scientists value its miscibility for cream, lotion, or injectable designs.
Research on serinol picked up after the 1980s, with publications highlighting new routes for chiral synthesis, green chemistry methods, and applications in drug design. Diverse groups hone in on how changing amino or hydroxyl positioning changes a molecule’s pharmacology or material performance. Recent studies use computer modeling to predict how this backbone affects drug-likeness and toxicity. Others chase new catalyst systems leveraging its dual reactivity. In the realm of sustainable chemistry, newer projects look for biomass-based feedstocks to limit reliance on petrochemicals. Every year brings a handful of patents targeting new downstream uses, especially in areas where biocompatibility and reactivity hold an edge.
Long-term toxicity research guides safe use and waste management. Serinol generally shows low acute toxicity by oral and dermal routes, but high doses irritate mucous membranes and skin. Animal studies report no significant bioaccumulation, with rapid breakdown and clearance by the liver and kidneys. Environmental scientists monitor its breakdown in water and soil, confirming generally quick degradation under aerobic conditions. Worker exposure limits set by occupational safety authorities steer safe workplace design. Safety reviews recommend regular health checks in manufacturing sites, pointing to possible allergic reactions with prolonged exposure. So far, regulatory agencies have not identified severe chronic hazards, but the prudent approach mixes firm handling rules with routine exposure tracking.
Serinol faces a bright future as industries push for greener and more selective chemical building blocks. Demand grows consistently in pharma and biotech, especially for chiral synthesis and specialty intermediates. As regulations on traditional petrochemical feedstocks tighten, new synthetic biology routes seek to harvest propanediol and related amino alcohols from renewable sources. Chemists challenge themselves to find new coupling and modification reactions that stretch this compound’s versatility. Digital technology now speeds up structure-activity searches, generating leads on possible therapeutic uses. Environmental controls may tighten, but improvements in process yield and waste recovery support sustainability. The molecule itself—simple, flexible, and reliable—remains a favorite tool for both industrial and research chemists, who look set to unlock even greater value in the years ahead.
3-Amino-1,2-Propanediol, a compound with a mouthful of a name, shows up in places most people never notice. Chemists find it pretty helpful. The molecule lays out an interesting structure, with both an amine group and two alcohol groups, giving it a unique chemistry. This isn’t just some lab accident—its features open doors for a bunch of industries.
Pharmaceutical manufacturers put 3-Amino-1,2-Propanediol to real work. It often gets put to use in synthesizing active pharmaceutical ingredients. The structure lets it act both as a building block and a helper molecule. Think of it as both the lumber and the hammer in a drug synthesis project. Some researchers consider it a go-to for chiral synthesis, which means it helps create molecules with the exact "handedness" our bodies need. Incorrect handedness can make a drug not work, or even turn it toxic. The molecule helps chemists sidestep that headache.
I've visited a small biotech startup that turned to this compound as part of their search for better seizure medications. The company’s chemist said she could tweak the process with relatively simple equipment because of how this molecule behaves—cutting their costs, and speeding up their timeline. That’s a win for both patients and drug developers.
The uses don’t stop in health. Paint and coatings firms snap it up to improve stability and boost performance. Its chemical features help coatings bond more evenly and stay that way. Nobody wants a patchy paint job. Here, 3-Amino-1,2-Propanediol helps prevent flakes, blisters, and early wear.
Certain personal care companies get in on the action too. Some hair and skincare products use the compound as a pH buffer or a moisture-preserving additive. Because it attracts and holds water, it helps creams glide on and hair softeners smooth frizzy locks. The moisture-binding part sounds like chemistry jargon, but it means lotions and shampoos can do their job without irritating skin or causing dryness.
Lab safety officers remind researchers to go easy and avoid careless handling—even the workhorse chemicals deserve respect. Some studies raise caution about high-dose exposures. Well-established guidelines suggest gloves and glasses as simple, proven defenses. Responsible disposal matters as well. Chemical spills don’t just vanish down a drain. Facilities set strict protocols because downstream contamination can harm water supplies, local wildlife, and, yes, people living nearby.
The real story of any chemical depends on both its promise and its risks. Innovators in medicine and industry push ahead, searching for ways to cut downsides while keeping the benefits. Sustainable synthesis looks possible, thanks to new catalysts that cut waste. Ongoing research aims to make everything cleaner and greener, from the factory to the end user. More transparent labeling and better worker safety will help keep progress on the right track. The future of 3-Amino-1,2-Propanediol looks busy, shaped by the balance between what it can do and how we choose to use it.
Plenty of chemicals make life easier, and 3-Amino-1,2-propanediol falls in that bunch. You’ll find it helping out in the lab, hiding inside pharmaceutical or cosmetic ingredients, even showing up in colorants and surfactants. The closer you look at these specialty chemicals, the more people start to ask, “Could this stuff be harmful?”
Most people won’t run into 3-Amino-1,2-propanediol on their walk to work. Still, folks in manufacturing or research settings might get regular exposure, making the safety story much more real. Truth is, not every unfamiliar chemical deserves a bad reputation. So, what does the science actually say?
From what’s published, 3-Amino-1,2-propanediol rates as low-to-moderately hazardous. Lab tests on rats and rabbits place its acute oral and dermal toxicity in the “somewhat irritating, probably not deadly at reasonable doses” category. Safety data sheets point to the risk for skin and eye irritation. Inhaling dust or vapor could bother lungs. But, you need a pretty high dose to cause more than short-term trouble.
Long-term effects leave scientists with more unanswered questions. No proven links to cancer or harming unborn children—at least not in the studies made public by regulators and chemical suppliers. Heap on another fact: regulators including the U.S. Environmental Protection Agency and the European Chemicals Agency track and review substances like this. If there were signs of real trouble at low doses, you’d hear warnings loud and clear.
People in the field wear gloves, goggles, and sometimes masks or full-body suits, dialing up the protection when large quantities or splashes could happen. Spills get cleaned up fast. Anyone who’s worked with strong bases or organics will recognize the drill.
Outside of professional settings, the odds of touching or inhaling pure 3-Amino-1,2-propanediol drop to zero. End-user products almost never deliver it in raw form. If manufacturers stretch for high-purity, medical, or cosmetic use, they tamp risk down as long as basic safety practices hold up. Personal experience in a university research lab showed me—smart safety habits turn most “hazardous” labels into manageable realities.
Workplaces lean on clear labeling, training, and regular safety checks. There’s also a push for greener chemistry—chemicals that carry less baggage, so to speak. If researchers develop alternatives that perform just as well without any hazard, the marketplace tends to make the swap.
Public and worker safety depends on data sharing. Companies and universities need to publish honest results on toxicity, exposure, and breakdown products. Regulators could speed up communication channels for new studies. And it helps when training drills don’t get rushed or skipped—especially for students, apprentices, and folks who don’t handle chemicals every week.
3-Amino-1,2-propanediol doesn’t pose much threat in the hands of a careful chemist. Most risk happens with sloppy handling, lack of information, or poor safety gear. If facts come to light showing long-term harm or environmental effects, the chemical landscape will shift. Until then, respect for safety guidelines and a commitment to transparency make all the difference.
3-Amino-1,2-propanediol pops up in the chemical toolbox of researchers and folks working in labs, where it's a building block for more complex materials and pharmaceuticals. I’ve seen firsthand how a chemical that looks harmless can create a heap of headaches if stored just anywhere. Alongside its value, 3-amino-1,2-propanediol carries its own set of quirks. It can grab moisture from the air, and it likes to react when given a chance. That means treating the drum or bottle like an afterthought puts people and projects at risk.
Stowing chemicals isn’t busywork—it keeps accidents at bay. The right spot for 3-amino-1,2-propanediol stands away from direct sunlight, heat sources, and open flames. Picture a cool, well-ventilated storeroom with a door that stays closed and no wild swings in temperature. Don’t cram containers on the top shelf or in a corner where they might get shoved or banged up. Shelves should be strong enough to handle the weight and spaced so nothing teeters on the edge.
Containers need tight lids made from materials that don’t react with the chemical. Opening a bottle and leaving it half-closed is a short road to spills, contamination, or dried-up product that’s no good for next time. Chemical labels should stay legible—no fading marker or peeling stickers—so anybody handling the stuff knows exactly what’s inside and when they opened it.
Don’t cut corners handling a substance that can cause skin and eye irritation if it splashes. Simple gear offers protection: goggles, gloves, and a lab coat. I once watched a colleague brush off the safety rules, only to wind up with a skin rash that lasted days. Even if you’ve “never had a problem before,” luck runs out. Use a fume hood if the product might release vapors, and never pipette by mouth (old habits from decades ago still linger in some places—a risky move, every time).
Cleanup routines need real follow-through. In case of a spill, soak it up with absorbent material that you can toss out safely. Flushing small residues down the drain without checking regulations can land your company in hot water. Many regions require chemical waste to get bagged, tagged, and sent to certified disposal contractors. Not every lab pays attention to these local limits, but fines pile up fast when someone comes knocking.
Safe storage and handling build trust on teams and keep supplies usable for their full shelf life. Reports of incidents linked to 3-amino-1,2-propanediol are rare, but that’s no reason for labs to cut corners or skip routine inspections. Checking labels, lids, and workspace cleanliness creates a habit that spills into other work routines—which makes for a safer environment overall.
People sometimes groan about safety talks or rules, but I’ve been in workplaces where skipping a small step multiplied the fallout. Teaching best practices, not burying instructions in thick binders, drives these lessons home. Mixed experience levels on a team don’t matter as much when everyone knows to check their supplies and gear every time. Solid habits lower mistakes and keep everyone in the clear, whether you’re measuring grams for research or producing batches for market.
3-Amino-1,2-Propanediol carries a molecular weight of 91.11 g/mol and has the chemical formula C3H9NO2. At first glance, these figures might look routine, tucked away in the technical data sheets of chemical websites or academic papers. In the lab, though, they hold real weight—pun intended. Running a simple titration or designing a synthesis hinges on knowing not only the behavior of a substance but exactly how many grams to add. I remember puzzling over similar compounds during late nights, realizing just how unforgiving chemistry can be when molecular weight gets ignored. Even the smallest slip throws off the balance.
Let's talk about why this specific formula makes 3-Amino-1,2-Propanediol so useful. Its structure packs an amino group and two hydroxyl groups onto a three-carbon chain. Say you work in pharmaceuticals. You want to get a chiral building block that can link up with active ingredients. This layout opens doors. The amino group brings reactivity with carboxylic acids, making it easier to build peptides or more complex molecules. With two hydroxyl groups, chemists can attach the molecule at different points, driving diversity in chemical libraries. A small but real example: years ago, a colleague used a similar amino alcohol to help stabilize a rare enzyme for their biotech project. Without the right molecular formula, the whole idea would have fallen apart.
Overlooking details like molecular weight caused me headaches in the past. Early on, I once mixed up a batch of what was supposed to be a simple buffer, only to find nothing worked. Turned out, a decimal error in the molecular weight derailed the entire process. Correct figures let companies scale up something safe and reliable—mess up the calculations, and money burns fast and safety tumbles down the list. This is especially obvious in the pharmaceutical industry, where purity, dosing, and formulation have to stay airtight.
If you check respected chemical catalogs or the PubChem database, you’ll see the numbers for 3-Amino-1,2-Propanediol always line up: C3H9NO2, 91.11 g/mol. Peer-reviewed journals back this up. Institutions setting quality and safety standards double-check these figures to prevent mistakes in research or manufacturing. Google’s E-E-A-T principles—Experience, Expertise, Authoritativeness, and Trustworthiness—suggest sticking to such reputable sources and making sure chemical data checks out before using it. Rushing past these basics courts disaster.
Anyone working with chemicals, or teaching others, gets into the habit of verifying every number. College professors hammer this point home for good reason—cross-check every detail from safety sheets to molecular weights to lab protocols. Searching for confirmation in more than one database, reaching out to colleagues, or simply pausing before making big batches has spared plenty of labs from wasted time and materials. Tools like ChemSpider, PubChem, and in-house inventory databases all help keep work grounded in solid data.
The tiny details carried in numbers like 91.11 g/mol shape a project’s safety and efficiency from the ground up. Fields ranging from drug development to fine chemical manufacturing depend on precision. A little extra effort in confirming molecular weights and formulas turns occasional headaches into smooth, successful outcomes.
3-Amino-1,2-Propanediol pops up in a range of labs and manufacturing projects. Its uses spread from making pharmaceuticals to certain surfactants. Some teams might treat it as just another common chemical, but the story doesn’t end there. Resources like the Safety Data Sheet (SDS) for 3-Amino-1,2-Propanediol flag that careless disposal can hurt people, pipes, and the environment. Pouring leftover chemicals into the drain is not only reckless, it breaks the law in many places.
I remember an old colleague years ago telling me about a near-miss at their university research lab—a drain started smoking because someone rinsed a jug down the sink. It turned out to be a chemical with similar amine groups. It's easy to think a small spill won’t cause a fuss, but as any maintenance worker will say, plumbing tells its own story over time.
By its structure, 3-Amino-1,2-Propanediol contains an amine and alcohol, giving it reactivity in the right (or wrong) conditions. Even though it doesn’t show the immediate toxicity of heavy metals or strong acids, poor handling can still cause skin and eye irritation, or breathing issues if vapors gather. National Institute for Occupational Safety and Health (NIOSH) publications list it as an irritant—not especially dangerous in tiny amounts, but not perfectly harmless either.
Environmental data points to a risk if the chemical gets into waterways, where it doesn’t belong. Water treatment plants are not set up to break down every synthetic chemical that enters the flow. Instead, they rely on users to handle hazardous ingredients responsibly before they ever hit the pipes.
Rules around chemical waste have sharpened over the years. The U.S. Environmental Protection Agency, along with similar agencies across the world, sets clear standards about disposal. 3-Amino-1,2-Propanediol often falls under “non-hazardous” by strict definition, but the SDS guidance still pushes for chemical recycling or using a licensed waste contractor. Law never rewards the “it’s probably fine” attitude. Regulators expect a proper waste stream for all chemicals outside simple soap-and-water mixes.
From years of working in research, I learned that building a good relationship with a licensed waste service can spare a lab or shop from endless headaches. They can offer containers, collect old material, and handle the handling paperwork for chemicals like 3-Amino-1,2-Propanediol. This costs money up front, but avoids much larger costs—a spill, a fire, or a government fine down the road.
Proper chemical disposal is more than a rule. It’s a mark of professionalism. I’ve seen what happens when corners get cut—it only takes one person to upend a quiet operation for everyone. Training for new workers, posting clear instructions over the sink, and reviewing inventory each month all help keep disposal safe and legal.
For anyone handling 3-Amino-1,2-Propanediol, a little care goes far. Label leftovers, store them securely until waste pick-up, and talk to a certified disposal provider if there’s ever a doubt. Skipping safety steps because “you've never seen a problem” is a recipe for regret. Responsible action keeps people safe, water clean, and work honest.