Chemists first isolated 2-Phenyl-1,3-Propanediol over a century ago, during a period of intense exploration into phenyl derivatives and diols that shaped much of organic chemistry’s growth. Early records in patent literature reveal that interest in this compound stemmed from its relationship to both propanediol and aromatic systems, promising a bridge between simple alcohols and more complex aromatic compounds. Across decades, curiosity about its reactivity and synthetic versatility gradually fed into more defined projects: pharmaceutical intermediates, specialty chemicals, and fine organic syntheses. As laboratories became more adept at manipulating aromatic chemistry, its place only solidified. My own experience in an academic setting impressed upon me how these ‘simple’ aromatic diols often appear in retrosynthetic pathways aimed at creating pharmaceuticals or even specialty polymers.
2-Phenyl-1,3-Propanediol stands out as a clear, colorless crystalline compound, most often recognized for its role as an intermediate in manufacturing processes. It boasts a straightforward structure, yet it’s precisely this quality—its dihydroxy propane backbone linked to a phenyl ring—that lends such utility. The material finds application in both research and industrial settings. On the shelf in the lab, it looks like a fairly unremarkable white solid, generally packaged in sealed, clearly labeled containers, ready for weighing and use in synthesis. Companies market it to academic researchers, chemical suppliers, and pharmaceutical firms alike, lending it a degree of familiarity among those who work with specialty organic chemicals.
This solid melts in the range of about 64–68°C, making it easy to handle but not so easily vaporized. Its molecular formula is C9H12O2 and the structure combines a benzene ring with a propanediol chain, which gives a molecular weight of 152.19 g/mol. Slightly soluble in water, it dissolves better in organic solvents like ethanol or diethyl ether, a characteristic trait of moderately polar aromatic alcohols. Under most storage conditions, it’s stable. The two hydroxyl groups create interesting hydrogen bonding opportunities, generally raising boiling and melting points compared to simpler phenyl derivatives. There’s a faint, sweetish odor—not unlike other aromatic alcohols. If you’ve weighed it out for a reaction, you’ll notice that it clumps a little in humid environments, showing its love for moisture.
Any reputable source supplies 2-Phenyl-1,3-Propanediol with labeling that details purity, batch number, and standardized handling codes. Purity runs at or above 98% in most commercial products, often with results for water content, trace solvents, or metals attached. Labels usually carry both the systematic name and recognized synonyms, plus safety codes matching current regulatory standards. Packaging varies by quantity: small glass bottles for milligram- to gram-scale, amber vials for light-sensitive storage, and bulk containers lined for chemical stability. Serial numbers and manufacturing dates should trace back to quality control records, which any supplier with good practice keeps on file. Regulatory compliance goes beyond labels, of course: accompanying papers flag any shipping restrictions, especially when heading across borders due to jurisdictional differences in chemical control.
Industrial and laboratory synthesis of 2-Phenyl-1,3-Propanediol most often starts from benzaldehyde and propylene oxide. A classic route uses benzaldehyde, which reacts with ethylene glycol in the presence of a catalyst to produce the diol by hydroxyalkylation. Another method involves reduction of 2-Phenyl-1,3-propanedione or related α,β-unsaturated ketones. Catalytic hydrogenation offers a reliable means to reduce precursors, especially for companies producing at scale. My internships in research-scale synthesis always taught that reaction monitoring—often by thin layer chromatography or NMR—can catch side-product formation early, keeping yields high and downstream purification manageable. Crystallization or distillation completes most syntheses, filtering away colored byproducts or unreacted starting materials.
2-Phenyl-1,3-Propanediol acts as a flexible platform for chemical modification. Both hydroxyls can undergo typical transformations, including esterification, etherification, or oxidation to aldehydes and acids. Attachment to resin supports or derivatization by tosylation opens new reaction channels, which synthetic chemists frequently use to build more complex molecules. The aromatic ring itself can undergo various electrophilic substitutions, if one first protects or otherwise manages the diol reactivity. Reduction and oxidation reactions need careful planning to avoid oxidizing both alcohols at once, unless such a transformation is the desired step. During my time researching lipid analogs, I saw how subtle changes on the phenyl ring or along the diol backbone yield new properties, creating variants with altered solubility, toxicity, and biological function.
This compound answers to several names, depending on supplier and application. Common synonyms are Hydratropic Glycol, 1,3-Dihydroxy-2-phenylpropane, and Benzenepropanediol. In research catalogs, expect to see English and sometimes translated names. Regulatory documents and shipping manifests might refer to its systematic IUPAC designation, with registry numbers provided for cross-reference. Trademarks do exist, mainly among companies specializing in pharmaceutical intermediates. Anyone searching for the compound should consider all these aliases—otherwise, important safety or regulatory information may go unnoticed.
2-Phenyl-1,3-Propanediol has a moderate safety profile. It carries standard risk statements for irritation to skin, eyes, and, to a lesser extent, respiratory tissue. Good lab safety protocols—lab coat, gloves, and local exhaust ventilation—address most routine risks. Safety data sheets explain precautions and disposal procedures in detail, including what to do in case of accidental spills, fire risk from dust, and emergency contact information. During benchwork, the solid doesn’t create much hazard, but improper storage—especially exposure to strong acids or oxidizers—may lead to unwanted chemical reactions or degradation. Packaging always follows chemical hygiene best practices, and staff routinely undergo safety refreshers. Shipping regulations treat it as a standard non-hazardous organic chemical, yet international guidelines (especially REACH in Europe) require full documentation.
The broad utility of 2-Phenyl-1,3-Propanediol centers on its role as an intermediate. Pharmaceutical syntheses rely on it as a scaffold for producing beta-blockers, anticonvulsants, and other bioactive molecules. Specialty polymers and plasticizers may also arise from modified versions of this diol. Some fragrances and flavors benefit from intermediates derived from this compound, though direct use in food or cosmetics stays rare due to limited toxicity data. Researchers in my own network have used it for enzyme studies—those two hydroxyls bind into active sites or mimic other biochemically relevant diols. Specialty fine chemical firms see steady demand for it, especially in markets that demand tightly controlled synthetic materials.
Ongoing research seeks to optimize the synthesis of 2-Phenyl-1,3-Propanediol, refining yields and minimizing byproducts, especially to meet environmental standards. Green chemistry goals point toward catalytic and solvent-free conditions, with some work focused on biocatalytic synthesis or renewable feedstocks. Structural analogs regularly appear in medicinal chemistry screens, as researchers test the effect of small changes on biological activity. Automation and digital workflow tools let smaller research groups experiment with new uses, since predictive modeling makes it easier to move from theoretical reaction to benchtop synthesis. My university labs frequently returned to this core structure in research proposals, reflecting both its reliability and the desire to find downstream applications in therapeutics or advanced materials.
Existing studies show low but not negligible toxicity for 2-Phenyl-1,3-Propanediol. Acute exposure leads mostly to mild irritation, though chronic effects lack thorough investigation. No strong evidence links it with carcinogenic or mutagenic effects, but regulatory bodies keep its use away from consumer products until further research fills these gaps. Some studies on related compounds suggest that metabolic breakdown creates benign products, but without clinical trials, safety margins remain conservative. Environmental fate studies raise moderate concerns; aromatic diols can persist if released in large quantities, though proper waste management in chemical industries reduces this risk. Advances in analytical chemistry should improve detection of trace environmental residues, aiding future risk assessments.
2-Phenyl-1,3-Propanediol stands at an interesting point for future development. Demand from pharmaceutical and specialty chemical industries shows no sign of slowing, especially as new therapies and polymers call for reliable intermediates. Regulatory pressure will likely drive innovation in safer, cleaner production routes. Advances in synthetic biology could unlock new routes—microbial factories engineered to produce aromatic diols might soon challenge traditional chemical synthesis on both cost and sustainable footprint. Research into analogs with enhanced solubility or selectivity could give rise to entirely new applications, from improved drug carriers to next-generation materials. As technology evolves, those who work closely with these fundamental building blocks will shape both products and practices to meet higher expectations in health, environment, and performance.
When talking about organic compounds with a complicated name, many folks think of distant lab work or academic circles. What many don’t see is how certain molecules, like 2-Phenyl-1,3-Propanediol, impact products we draw on daily. Scientists often call it PPD for short. This substance shows up most often as a chemical intermediate, which means it plays a key role in making other valuable products.
Pharmaceutical manufacturers find 2-Phenyl-1,3-Propanediol useful for building more complex drugs. Imagine you’re designing a medication used to manage chronic pain or anxiety. Some types of muscle relaxants, and certain anticonvulsants, have relied on structures similar to what this molecule provides. Its unique shape offers a solid skeleton for crafting drugs. I remember reading about chemists using it to develop analogs of drugs like carisoprodol. By modifying its structure, researchers have navigated both effectiveness and safety, trying to strike the right balance to reduce side effects or unwanted dependencies.
This compound doesn’t stop in pharmaceuticals. It crops up among specialty chemicals, too. Take fragrances or personal care products. While not a final ingredient for rugs or face creams, it lends a backbone for building blocks that create gentle scents or solvents. The way this molecule holds its alcohol groups makes it a key candidate in producing plasticizers, which give plastics some flexibility. I’ve met chemical suppliers who’ve shared stories about shifting production lines to handle derivatives of compounds like PPD just to keep up with growing demand for new materials or safer ingredients.
Using chemical intermediates in consumer and pharmaceutical manufacturing brings responsibility. Safety must always take center stage. People working with or around 2-Phenyl-1,3-Propanediol need to understand how to handle it. Material safety data sheets recommend proper ventilation, gloves, goggles, and storage protocols. Regulatory agencies check these standards because exposure, even to smaller production byproducts, can raise real risks. I once sat through a regulatory training where supervisors stressed tracking each chemical’s journey from delivery to disposal.
Environmental responsibility means thinking about a chemical’s full lifecycle. The world demands greener manufacturing. This challenge forces chemical companies to design routes for synthesizing 2-Phenyl-1,3-Propanediol with fewer toxic reagents and lower waste. In my experience talking to process engineers, many now study how biocatalysts can swap in for harsh metallic agents. This method lowers the burden on wastewater plants and cuts down hazardous residue.
Real change boils down to teamwork between scientists, policymakers, and the public. Honest communication about what’s in our products, why these chemicals matter, and how they’re controlled helps everyone. Transparency earns trust. If companies invest in safer practices and regulators support easy-to-access information, consumers stand to benefit. In the everyday hustle, most don’t pay much attention to chemical names, but they do care when safe products reach their shelves and medicine does its job without surprise side effects.
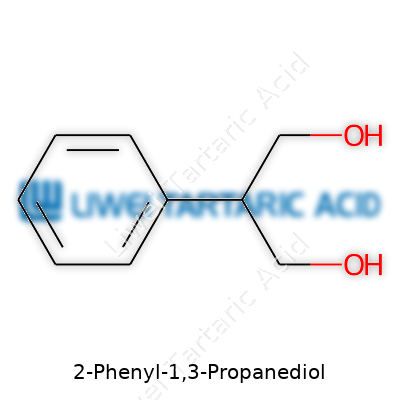
2-Phenyl-1,3-propanediol sounds complicated, but its shape keeps things interesting. The molecule branches out from a three-carbon chain, with two alcohol groups holding tight on the first and third carbon. A benzene ring hangs off the second carbon, bringing a little attitude from the aromatic side of chemistry. To visualize it in simple terms: HO–CH2–CH(Ph)–CH2–OH, where "Ph" stands for that six-carbon benzene ring.
The setup of two alcohol groups on the ends, plus that aromatic ring right in the middle, does more than shape the way chemists talk about it. These diol groups love to make hydrogen bonds, and that means 2-Phenyl-1,3-propanediol mixes well with water. Strong bonds like these open up doors for use as a building block in medicine, especially in drugs that require a certain balance of water-loving and water-repellent pieces. For a practicing chemist, this diol helps piece together bigger molecules without much fuss, and labs often rely on this type of structure for custom syntheses.
Back in grad school, I saw classmates experiment with 1,3-propanediols for new polymer types—a real-world struggle that gave me some serious respect for small changes in molecular skeletons. Add that phenyl group to the center, and suddenly you’ve shifted melting and boiling points, and even managed to alter solubility in ways that make or break a research project.
Big names in pharmaceuticals, polymers, and specialty chemistry value ingredients like this one. The benzene ring isn’t just decorative—it can stack with rings in biological molecules, leading to better fit or stronger action for products that end up in skin creams, pills, and more. Its structure enables innovation, and companies test out modifications to chase better shelf life, easier manufacturing, and reduced unwanted reactions.
Combination is the name of the game in chemical synthesis, and 2-Phenyl-1,3-propanediol lets manufacturers engineer new molecules. Processes including esterification and etherification depend on the strength and location of those hydroxyl groups. In personal experience working with formulation scientists, I’ve seen how this diol can act as a starting point—or "scaffold"—for medical compounds and some high-durability plastics. When every molecule counts, small differences matter.
Production scale brings up questions of purity. Small impurities in starting materials can tangle up syntheses, cause side reactions or even threaten performance in final products. Labs that produce this compound for high-purity work invest heavily in purification steps, from recrystallization to chromatography. Regulatory requirements push for thorough documentation, which often slows things down but ensures product safety.
Another challenge involves sustainability. Traditional methods of producing aromatic compounds depend on fossil-based feedstocks. Shifting toward greener synthesis, maybe even bio-based benzene alternatives, gives this compound a chance to play a role in the push for eco-friendly chemistry. Some startups experiment with renewable approaches for diol synthesis, though cost keeps big factories from making the switch overnight.
This isn’t some forgotten entry in a chemical catalog—2-Phenyl-1,3-propanediol keeps showing up in research and industry because its structure packs a punch. Chemists like me keep an eye on tweaks to make the compound safer, greener, and better at what it does. It’s a clear example of how a little organic chemistry insight feeds directly into real-world products that touch lives every single day.
Working with chemicals like 2-Phenyl-1,3-Propanediol, you pick up a lot of respect for small, often invisible risks. Many labs hint at safety on the wall charts, but the real lessons stick after a few close calls and late-night cleanup shifts. This compound isn’t the most notorious in a chemical cabinet, but it deserves genuine care. Even those who only need to handle it occasionally benefit from running a tight safety ship.
A pair of nitrile gloves beats bare hands every time chemicals get involved. Washing up doesn’t undo skin exposure, so gloves stay on from start to finish. Splash goggles do more for peace of mind than most realize—one stray droplet in your eye shifts a day from routine to panicked scramble pretty fast. I’ve seen people skip the goggles, but it’s not worth the risk. Lab coats and closed shoes keep drips from leaving marks you’ll regret.
The smell of solvents still reminds me of late evenings sorting glassware under the hum of a working fume hood. 2-Phenyl-1,3-Propanediol doesn’t rank high for volatility, but fumes shouldn’t go unchecked. Letting vapors build up feels like courting trouble, especially in smaller workspaces. Fume hoods and open windows aren't decoration; they're basics for long-term health and keeping the air clear of anything you don’t want to breathe.
Most problems with chemicals happen not from malice, but rushing or forgetting details. Storage makes a difference—tight lids, dry shelves, and clear labels turn chaos into order. Mixing up bottles or leaving caps loose just isn’t worth the cost. At one job, someone poured an unknown liquid down the sink, thinking it was water. The scent alone should have warned them. Proper disposal means using chemical waste containers, not the public drain or regular bin.
Trusted sources like PubChem and manufacturer safety data sheets point out that 2-Phenyl-1,3-Propanediol may cause irritation to skin and eyes. It shouldn’t get inhaled or swallowed. These resources don’t exaggerate; minor irritation now can turn into allergies or longer-term hassle. The American Chemical Society and Occupational Safety and Health Administration both remind workers not to wait for symptoms before acting safely. For those who want specific details, OSHA’s Hazard Communication Standard acts as the foundation for sharing risks in workplaces.
Reading safety sheets isn’t enough. A spilled beaker at the wrong moment tests any prep. Real training—taking time to practice drills, knowing where the eyewash is, being able to grab a spill kit with your eyes closed—matters more than flashcards. I’ve been in rooms where panic triumphed over common sense, but knowing the drill makes those moments easier. First aid supplies belong nearby, not locked away. Eye washes, showers, and cleanup materials should always stay accessible.
Safe handling grows from a workplace culture where people call out shortcuts gently, where no one acts embarrassed to double-check a label or glove up mid-task. In my experience, the best teams look out for one another’s health—not just their own. Handling 2-Phenyl-1,3-Propanediol by the book protects not only the handler but everyone who shares that toolbox or lab bench.
Many who work in fine chemicals or specialty formulations understand the importance of reliable purity for 2-Phenyl-1,3-Propanediol. Your end result depends on how clean your starting materials are. Most labs and factories lean toward grades offering 98% purity or higher. That level comes as a result of careful crystallization or distillation, not luck. More demanding applications—analytical research or synthesis of pharmaceuticals—go with 99% or above, since even tiny impurities can skew reactions or test results.
My time supporting small-scale synthesis taught me not to cut corners with purity. Contaminants can sneak in and ruin more than a single batch—they set off extra troubleshooting, lost hours, and blown budgets. The chemical trade often supplies certificates of analysis, with HPLC or GC-MS traces. These aren’t fancy paperwork—they confirm what’s actually in your drum or bottle. Without clear traceability and transparency, bad batches make their way into production, and quality drops across the board.
Small research operations usually pick glass bottles, ranging from 25 grams to 500 grams. Glass works well—it guards against air and moisture, and lets you see what’s inside. Out in industry, folks order in bulk, so HDPE jugs or composite fiber drums show up more. These containers resist corrosion, keep out sunlight, and simplify stacking in cramped storage. You get anywhere from 1 kilogram to 25 kilograms, sometimes even up to 200 kilograms in closed drums if you’re running a larger facility.
Shipping regulations shape design decisions. Flammable or moisture-sensitive chemicals pack in UN-approved containers, which often look like simple drums but follow rigorous drop, leak, and compression tests. Only recently have I seen some vendors offer vacuum-sealed or nitrogen-flushed pouches, which keep the material from picking up water or oxidizing, especially on long ocean journeys. That’s a sign that both the supplier and the end user care about batch stability, not just what’s on paper.
Most people pick their packaging based on lab protocol or warehouse space, but not everyone thinks hard about safety. 2-Phenyl-1,3-Propanediol doesn’t rank among the most hazardous, but dust or spills are real issues. If you’ve ever tried to clean up a wide-mouth drum spill, you know a little mistake can wreck hours of work and create extra paperwork. Good packaging choices reduce risk—tight seals and drip-proof lids mean fewer accidents.
Regulatory review keeps most suppliers on their toes. Countries in North America and the EU both require clear hazard labeling. I’ve run into shipments blocked at customs over missing or faded labels, so I always stress to colleagues to double-check before shipping out. Tamper-evident seals and QR codes for batch tracking are popping up more, a reflection of how traceability is now viewed as non-negotiable.
A lot of the frustrations in supply chains boil down to poor communication between supplier and user. Some buyers still focus on lowest price and forget to ask for purity documentation or reliable packaging. Companies that foster direct dialogue and routine quality checks raise the bar across the industry. Whether you’re buying a single bottle for lab use or a pallet for a production line, insist on full transparency. Keeping open records, accurate labeling, and picking packaging that guards your material pays dividends in reduced waste and safer workplaces.
A lot of inquiries land in specialty chemical markets about 2-Phenyl-1,3-Propanediol. Someone might see it referenced in a publication about pharmaceuticals or polymer synthesis and get curious—can you buy this stuff in bulk? On paper, it looks straightforward. In reality, availability gets tangled up with tight regulations and low production demand.
I’ve talked with several procurement specialists in the chemical sector who know the struggle to source relatively obscure diols. Popular glycols, like ethylene glycol or propylene glycol, don’t compare. 2-Phenyl-1,3-Propanediol occupies a much narrower market. The value of this compound connects to its unique double alcohol structure beneath a phenyl group—features attractive for fine chemical synthesis, maybe some research on CNS-active drugs, or advanced materials work. Most folks referencing this compound signal interest in synthesis pathways, not mass-volume consumer applications.
A big chunk of online “suppliers” just act as brokers. You only realize this after asking for a COA, safety data, or pricing for 25 kg drums. True producers often operate out of China, India, and Germany—regions with strong specialty pharma, research, and custom chemical sectors. Large distributors, including Sigma-Aldrich, TCI, and Alfa Aesar, sometimes hold only grams or a couple hundred grams on the shelf. Trying to purchase hundreds of kilos? That becomes custom order territory, quoted case-by-case, and usually needs direct negotiation.
Custom synthesis companies step in if the regular stock won’t cut it. I’ve seen firms like Synchem or Chemieliva field requests for 2-Phenyl-1,3-Propanediol as a custom batch. Timelines stretch to months and may involve minimum order sizes that can scare off newcomers. Costs skyrocket, too: If gram lots already fetch three-figure prices, kilogram scale brings sticker shock. That doesn’t even touch shipping, import duties, or the need for a detailed end-use declaration—the sort of paperwork EU and U.S. regulators expect these days.
Labs attempting to source this compound face a slew of paperwork. Agencies want to know if you’re using it for pharmaceutical research, specialty plastics, or something less legal. Different countries classify aromatic diols differently—some see them as harmless research chemicals, others tie them to more controlled categories. Failing to produce a justifying use case often spells the end of a deal.
Quality matters, too. Many chemists have a story about buying from a new supplier, only to open a drum and smell strange off-flavors or see unexpected coloration. High-purity 2-Phenyl-1,3-Propanediol is not guaranteed from every seller. Any impurity, even a percentage point, can ruin a batch. A better supplier will present a full certificate of analysis, sometimes NMR data, plus ISO certifications for their process. I’ve seen groups switch vendors three or four times before settling on a reliable source.
The best approach starts with a clear idea of required purity, volume, and timeline. Skip the “contact us for price” middlemen by looking up actual chemical producers in trade portals or by contacting established specialty chemical manufacturers directly. Do your compliance homework early—prepare intended-use explanations, import licenses, and personal references if the supplier requests them. Building a direct connection with production-side sales staff speeds things up, especially for custom runs.
Buying 2-Phenyl-1,3-Propanediol in bulk turns into a lesson in supply chain realities—high cost, heavy compliance, and a search for reliability rather than bargain hunting. Smart buyers share information, audit vendors, and keep backup suppliers on file. That’s how you get it done in the real world.