Chemistry always keeps moving, and the story of 2-Methyl-2-Propyl-1,3-Propanediol reflects that. This chemical didn’t emerge from a sudden eureka moment; it arrived through years of tinkering and advancing synthetic routes. Early on, research focused on improving molecular structures to serve a range of industries like pharmaceuticals and specialty lubricants. Over time, laboratories swapped guesswork for targeted synthesis, catching the eye of manufacturers who saw practical value in its stable form. Advances in purification and catalyst use during the late twentieth century helped make production more reliable and scalable, which opened up options for both specialty and large-scale providers. Seeing activity in patents and trade literature marked an uptick in application-driven research, linking academic curiosity with the needs of chemical suppliers, so historical momentum keeps adding new chapters to its story.
It’s rare for a molecule to be both structurally simple and useful, but 2-Methyl-2-Propyl-1,3-Propanediol falls into that lucky group. Folks in the lab see its dual hydroxy groups as prime targets for modification, which means it fits into a surprising number of formulations. You’ll spot it described as a raw material for solvent blends or as a building block for resins and plasticizers. Physically, it’s usually found as a colorless, viscous liquid—easy enough to handle in most facilities. Chemists appreciate that it doesn’t evaporate or degrade under typical storage because this signals fewer headaches during transport or scaling up. Its reactivity makes it a familiar face in synthetic chemistry. What helps most is that it plays well with many different reactants, so researchers keep it stocked near the workbench.
Measured up close, 2-Methyl-2-Propyl-1,3-Propanediol offers a boiling point in the range of 240–250°C and a melting point above 50°C. Its low volatility is an advantage, especially when handling large drums on a factory floor, meaning you don’t catch a whiff of evaporation at every turn. The density hovers around 0.95 g/cm³, which lines up closely with other small diols but makes it less prone to phase separation in multi-component blends. Water solubility opens up uses both in aqueous and non-aqueous settings. Chemically, the alkyl groups on the backbone provide a measure of resistance against oxidation—helpful if you want a product to last on the shelf or hold up under reactive processing.
Producers rarely cut corners with this compound, making purity a prime selling point. Material safety data sheets usually report purities of 98% or higher, with low water and organic impurity content. Labels commonly display the compound’s structure, batch details, and manufacturing date for traceability. Storage advisories tell staff to cork it up in a cool, dry place, since even with its stability, moisture or sunlight can gently nudge oxidation over time. On the topic of labeling, regulatory compliance forms part of every shipment—hazard identification, recommended handling gear, and exposure limits, all spelled out clearly so even new team members understand what they’re dealing with.
Industrial-scale synthesis starts with alkylation or reduction routes, often relying on protected starting materials to avoid side reactions. One process uses 2-Methyl-2-Propyl-1,3-Propanedione as the precursor and reduces it through catalytic hydrogenation, handing the final diol form quickly and with efficiency. Some routes involve Grignard or organolithium intermediates, chosen according to available feedstocks or cost. After synthesis, standard purification includes vacuum distillation followed by filtration or solvent extraction to reach above 98% purity. Operators stay alert to reaction exotherms, since careful temperature control makes the difference between a smooth batch and wasted raw materials. Labs and factories stay nimble by modifying processing steps for new applications or changing government import rules.
Every chemist I know enjoys the flexibility of compounds like 2-Methyl-2-Propyl-1,3-Propanediol. Each hydroxy group can take on a wide variety of substituents. For example, alkylation and esterification turn the base molecule into derivatives for use in higher-value specialty chemicals. Reacting with acid anhydrides forms esters; these can carry lubricity or solvent properties into final products for everything from adhesives to coatings. Chemists sometimes convert those hydroxy sites to halides or ethers, providing even more possible branches in downstream synthesis. Modification rarely ends there. Formulators even chain the molecule into polyols for making thermosetting polymers, showing the compound keeps unlocking more use-cases as research budgets—big or small—allow.
Few chemicals dodge the name game, and this one is no exception. Depending on context, you’ll find 2-Methyl-2-Propyl-1,3-Propanediol listed under alternative names like MPD-2 or its CAS descriptor, though exact product names can shift between regions. Sometimes I come across trade names assigned by big suppliers; these often reflect marketing efforts to boost recognition in crowded industrial catalogs. For patent filings, full IUPAC names show up, but in R&D circles, shorthand versions rule the day. No matter the tag, up-to-date documentation ensures distributors and researchers minimize confusion and mix-ups at the laboratory bench.
Nobody in the chemical industry shrugs off safety with this type of molecule. Standard practice means gloves and goggles every time. Workers get trained on procedures for spills, inhalation, and dermal contact; not because this diol is uniquely dangerous compared to other organics, but because exposure laws—like OSHA’s—insist on thorough regard for every potential hazard. While the compound isn’t as volatile or acutely toxic as many process chemicals, its relatively low flash point still demands storage away from open flames or heat sources. Ventilated storage reduces risk of build-up in the air. Facilities keep material data sheets at hand, running drills for emergencies and providing quick access to eyewash stations. Just as important, batch traceability from processing to packaging rounds out safety best practices, helping investigators pin down issues fast if workers ever report a problem.
I’ve seen 2-Methyl-2-Propyl-1,3-Propanediol appear in places ranging from coatings factories to electronics labs. The resin and plasticizer industries use it to tweak flexibility and toughness in finished products. It slides into paints and adhesives as a co-solvent or intermediate. Pharmaceutical chemists find uses in drug synthesis, either as a direct reactant or managed solvent for difficult reactions. Those in polyurethane chemistry like its dual functionality for network formation, strengthening foams and elastomers without introducing extra impurities that might cause performance issues. Lubricant manufacturers, ever focused on oxidative and thermal stability, also blend in this diol to boost longevity. It’s not a stretch to say nearly every applied chemistry sector has at least poked at its versatility.
Research keeps shifting as teams dig for new angles. Academic groups explore greener routes to this molecule, aiming to cut down on energy input or hazardous reagents. Industrial players dangle funding for next-generation applications—think biodegradable plastics or functionalized surfaces that self-clean under the right light. Researchers experiment by tweaking molecular modifications, chasing after properties like lower toxicity or greater reactivity for custom niche uses. The move toward automation and AI-driven synthesis has made accurate property and reaction prediction more important, and so datasets built around compounds like this one help guide future R&D. Everyone involved keeps watching regulatory cues as well, especially with tightening controls on chemical emissions and workplace safety.
Toxicology studies look for patterns in acute and chronic exposure. Results often place 2-Methyl-2-Propyl-1,3-Propanediol well below threshold levels of harm, with high LD50 values in animal models and limited evidence of irritancy in skin and eye contact at operational concentrations. That said, researchers don’t declare blanket safety. They check for metabolites and long-term effects in ecological systems, recognizing that what’s harmless in isolation can become an issue down the drain or in waste streams. The drive for transparency means that full findings—including negative results—get published, allowing regulatory agencies to revisit exposure limits when fresh data arrives. I’ve seen a steady push for alternative test systems, shifting some focus toward in vitro assays and computational prediction as science keeps questioning assumptions from past decades.
Looking ahead, it’s clear 2-Methyl-2-Propyl-1,3-Propanediol won’t fade away from supplier shelves or research plans soon. Its track record for dependability encourages further exploration in sustainable chemistry—bio-based production stands out as a hot topic. Industry wants to add more value by designing smarter derivatives that solve very specific application problems, from next-generation polymers to medicinal delivery vehicles. Advances in process engineering may deliver lower-cost synthesis and improved environmental footprints, as renewable feedstocks take on a larger role. Regulatory shifts toward safer workspaces and end-of-life disposal also set a challenge for both producers and end-users to rethink product life cycles. Whatever comes next, both industry and academia have compelling reasons to keep investing in its evolving story, making the next round of innovation just as interesting as the shifts of the past.
2-Methyl-2-Propyl-1,3-Propanediol doesn’t roll off the tongue, but in the lab and on the manufacturing floor, it stands out for its versatility. It shows up as a colorless liquid, barely giving off a smell, and slips into formulas in a way that makes it hard to notice unless you know what you’re looking for. Its structure brings flexibility to chemical synthesis, offering an alcohol backbone with branches that make certain reactions smoother or more efficient.
The most recognizable role of 2-Methyl-2-Propyl-1,3-Propanediol turns up in the development of muscle relaxants, especially carisoprodol and meprobamate. Both of these drugs have claimed spots on pharmacy shelves for decades. Chemists pick this diol because its structure fits well during the synthesis steps. Drugs that help people manage pain and muscle spasms rely on high-purity ingredients, and without this compound, a lot of common muscle relaxant pills wouldn’t exist in their current form.
It also finds a home in the world of specialty coatings and lubricants. Certain coatings need ingredients that can spread smoothly and resist breaking down when exposed to light, pressure, or water. 2-Methyl-2-Propyl-1,3-Propanediol brings hydrophobic (water-resistant) properties and provides a base that acts as both a solvent and a stabilizer. In my work with industrial chemists, there’s always chatter about how switching a base material can solve unexpected problems, like a coating turning cloudy after a few weeks or sticking unevenly to surfaces.
Anyone who works in cosmetics knows keeping a cream soft and stable can get tricky. 2-Methyl-2-Propyl-1,3-Propanediol helps as a moisturizing agent and stabilizer, especially in creams or serums meant to last all day or survive the rigors of shipping. It improves the “spread” feel of products, which makes a cream go on smoother or a gel dry faster. Durable, consistent products build trust and maintain the integrity of a company’s brand.
Every chemical introduced into a factory or a pharmacy needs proper safety data. Overuse or careless waste management threatens both workers and the environment. Studies point out low acute toxicity for this compound, but facilities still must treat it like other industrial chemicals: gloves, goggles, and well-ventilated spaces. Spills might not grab headlines, but I’ve seen how even minor mishandling can cause headaches for local water treatment or require extra cleanup steps. The modern push is toward “greener” synthesis, and companies are exploring ways to recycle or recover unused material to keep costs down and impacts low.
Greater transparency always leads to better practices. Manufacturers should share more about the sourcing and disposal of 2-Methyl-2-Propyl-1,3-Propanediol. Third-party audits and product labeling could help customers trust what’s in their medicine and personal care items. Research into plant-based alternatives or improved waste capture technology looks promising. Keeping the focus on responsible use means future generations of chemists will have fewer messes to mop up—with safer workplaces and safer products for everyone.
2-Methyl-2-Propyl-1,3-Propanediol sounds like something pulled out of a lab that most folks never hear about—unless you’ve spent time reading ingredient lists or scientific labels. Lab workers, students, and parents reading ingredient panels want to make sense of these complicated names and, more importantly, whether they are safe to be around or in. Facts matter when it comes down to what touches the skin or ends up in food.
Manufacturers use 2-Methyl-2-Propyl-1,3-Propanediol mostly for its solvent properties. It shows up in things like perfumes, cosmetics, and sometimes industrial cleaning agents. Some researchers have thrown it into the mix during testing for new drugs because the compound helps dissolve ingredients so they blend together. If you work in a plant, on a packing line, or behind a counter selling cosmetics, there’s a good chance this chemical has passed through your hands at some point.
This is one of those chemicals that hasn’t collected a long resume of human studies. Regulatory agencies, including the European Chemicals Agency and the US EPA, have only limited animal toxicology data. No recognized food safety authority lists it as safe for eating. Inhaling high concentrations in the workplace or touching the skin for extended periods leads to irritation in test results, and there’s no approved daily exposure amount for food or direct human use.
Chemistry degrees can lead you to spend time in crowded labs where spills and splashes happen. Everyone wears gloves and goggles around solvents because guessing safety based on smell or appearance is risky. Anyone who has dealt with industrial chemicals comes to respect labels that warn about irritation or unknown risks. Feeling your skin itch from an unknown solvent is sometimes all the proof people need to keep it away.
A neighbor once called after a factory leak made the news. He wanted to know if the odor in the air was safe for his kids. When ingredients are not food-approved or have incomplete safety data, common sense says avoid them unless you’re sure. I tell friends that if the label uses a long scientific name that doesn’t appear on a food-safe list or a reputable ingredient safety database, it’s not something to eat, drink, or use on skin unless necessary.
Growing up in a manufacturing town, the local clinic always pushed safety training and quick access to Material Safety Data Sheets. If a compound like 2-Methyl-2-Propyl-1,3-Propanediol lacks a clear green light for food or personal care use, companies and families should treat it with caution. Wear gloves and goggles during work, keep food and drink far from work areas, and ask supervisors about substitution if you feel worried about exposure.
Looking past legal requirements, the best rule is to trust trusted sources. Check databases like PubChem or the European Chemicals Agency record for new findings. Community voices can ask for clear disclosure and less reliance on doubtful ingredients, pushing companies to make things safer. For now, the science behind this compound doesn't give anyone a good reason to take risks with direct contact, especially if there’s an alternative available. Safety takes teamwork, both in factories and at home.
Chemistry isn’t always about long names and complicated formulas from textbooks. Sometimes it’s about breaking something down and figuring out what makes it tick. 2-Methyl-2-Propyl-1,3-Propanediol, a mouthful at first glance, actually packs a lot of useful details into that string of words. Working in a lab, I’ve picked up more from drawing out carbon chains by hand than any lecture could have taught me.
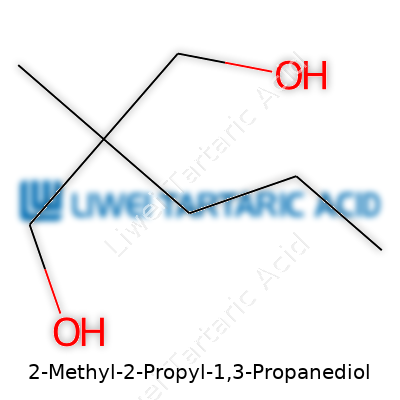
Every part of a name in organic chemistry hints at the molecule’s makeup. Here, “propanediol” tells you there’s a three-carbon chain that ends with two alcohol groups (-OH) stuck on carbons one and three. “2-methyl” and “2-propyl” mean the middle carbon—carbon number two—sprouts a methyl group (a little offshoot of one carbon) and a propyl group (three carbons in a row).
Line that up and you’ll get a backbone shaped like this: HO-CH2-C(CH3)(C3H7)-CH2-OH. If you count up the atoms, the molecular formula lands on C7H16O2. There’s something honest about seeing formulas spelled out. They don’t hide behind jargon or industry speak—they show exactly what sits on the bench.
Chemists look for certain features in a compound. With two alcohol groups, this diol shows strong hydrogen bonding. That lets it dissolve pretty well in water and hold its shape in a bunch of harsh conditions. Research and industrial teams can harness those traits, whether crafting new plastics, designing specialized lubricants, or tweaking pharmaceuticals.
I’ve often worked with polyols like this one in the lab when testing out new synthetic pathways. Their branching patterns change how they fit together with other molecules. The presence of both methyl and propyl branches around the central carbon creates a kind of chemical “shield,” which can guard against quick breakdown. That stability can be crucial, especially if the end product needs to last longer or perform under extreme conditions.
The field expects accuracy. Industry safety guidelines and regulatory documents always look for real chemical structures, not just common names or substitutions. Regulatory agencies and researchers both check the layout of a molecule to spot possible hazards, to make sure end products will not surprise anyone with unwanted effects. Back when I trained new lab staff, we always double-checked these diagrams before starting any synthesis. One mistake in the drawing could mean weeks of lost work or, worse, something unsafe.
Having the exact structure and understanding how every branch plays a part helps teams fine-tune reactions. By recognizing the small differences between molecules like 2-Methyl-2-Propyl-1,3-Propanediol and similar diols, chemists select the right tools for a job, push research beyond trial and error, and boost reliability. Small changes in branching can shift melting points, resistance to acids and bases, or even turn a material from soft and rubbery to strong and tough.
Maintaining this level of detail lines up with good research practices and keeps scientific work credible and safe. The drive to get it right comes from years of hard lessons at the lab bench. Every formula matters, every line in a structure model serves a purpose, and a thorough understanding leads to genuine progress.
Ask anyone who manages a chemical storeroom: shelf life means nothing if storage practices get sloppy. 2-Methyl-2-Propyl-1,3-Propanediol might not be a name you hear every day, but it shows up in a surprising number of lab and manufacturing settings. This substance has a reputation for stability, and folks get lulled into thinking it doesn’t need much thought. That’s a mistake. Anyone handling bulk raw materials knows that “good enough” turns into lost inventory or worse, safety headaches that creep up over time.
It’s easy for labels and lids to go ignored, especially when distractions pile up. I can recall stretches of my own lab work where a quick spot-check of containers after a shipment saved me from a much larger mess down the road. 2-Methyl-2-Propyl-1,3-Propanediol likes a dry, cool place. Moisture, direct sunlight, and temperature extremes will break down the quality and wreck your yields. Keep this chemical in an airtight, clearly labeled container—glass or high-quality plastic. Storage shelves ought to keep everything above floor level, and the room should stay low in humidity.
Reports don’t often chalk up big disasters to this particular compound, but skin and eye contact still call for protection. Gloves and splash goggles matter here. Spills grow into a problem if they happen near heat or acids. Absorbent pads and clear protocol make for quicker cleanups. In college, a friend tried to shortcut spill cleanup and ended up with a chemical burn. He learned the hard way: quick wipes with a paper towel only spread the problem around. Keep neutralizing agents and plenty of water close by—true for a lot of chemicals, but especially those you rarely think about.
Breathing in powder or vapor over time adds up. Ventilation needs attention—cracked windows don’t cut it. Basic respirators provide a solid line of defense, especially during transfers. That’s where people get lax: pouring between bottles or prepping samples. I’ve seen short staff shifts tempt folks to hustle past these steps. Training sticks with people long after manuals gather dust, so repeat sessions and real walk-throughs make long-term sense.
Mainstream safety often gets framed as a job for regulators, but most improvements start at the ground level. Easy-to-read signs, routine inventory checks, regular audits—nothing flashy, just solid habits. Clear communication channels allow everyone from interns to senior staff to flag issues. If something feels off, it probably is. The more eyes you have on storage conditions, the better.
Proper practices around 2-Methyl-2-Propyl-1,3-Propanediol don’t call for high-tech solutions. The old-school checklist and a culture that values small daily actions give everyone a better shot at keeping risk down and performance up.
Everyday life involves hundreds of chemicals, most with complicated names that seem far removed from daily experience. 2-Methyl-2-propyl-1,3-propanediol is another name that rarely pops up over the breakfast table, but it shows up in labs, industrial processes, and niche research fields. For people working in science or chemical manufacturing, safety questions always surface before a new substance hits the bench. Does this stuff cause harm? Does it build up in the body? Can a whiff send someone to the nurse?
Most safety data for 2-methyl-2-propyl-1,3-propanediol comes from animal studies or direct lab experience. Eye and skin irritation tend to arise as the top issues with direct contact. Lab technicians have reported redness, itching, or minor burns after spills, especially if no gloves or eye protection were involved. The chemical doesn’t release strong fumes at room temperature, so inhalation carries low risk in most workspaces, though dust particles can always irritate the lungs if someone is working with a fine powder.
Acute toxicity studies in animals show that high doses can cause sleepiness, decreased activity, and in some cases, trouble breathing. Doses used to cause these problems often far exceed what a person might experience accidentally. There’s no solid evidence of cancer or birth defects according to current published studies. Regulators haven’t flagged it for long-term dangers, but industry prefers to err on the side of caution until more is known, especially since many chemicals only reveal their nastier sides after years of use.
One big lesson from decades of chemical safety work—nothing stays quiet forever. Industrial solvents and additives from the past turned up in drinking water, dust, and all sorts of strange places. Persistent exposure, even at low levels, sometimes causes problems scientists didn’t expect. 2-methyl-2-propyl-1,3-propanediol flies under the radar in household products, but tighter regulation could follow if environmental or health watchdogs connect it to bigger issues down the road.
Simple safety rules cut risk. Gloves, goggles, and good ventilation act as the adults in the room. Chemical fume hoods, regular handwashing, and safe disposal go a long way to prevent accidents. I spent years in a chemistry lab, and colleagues who got careless eventually paid the price. Minor exposures might cause only a rash, yet repeated slip-ups turn into chronic problems. Good habits stick—and keep people working rather than sitting in a clinic.
People who never step foot in a lab likely won’t cross paths with 2-methyl-2-propyl-1,3-propanediol at home or in the office. Chemical plant workers, lab techs, and researchers bear the most risk. Proper training on material safety data sheets, spill response, and correct labeling keep teams out of trouble. Companies with solid health and safety culture often see fewer incidents over time; managers who support open reporting and regular safety meetings nearly always have healthier, happier staff.
Gaps still exist in long-term studies for this chemical. Regular monitoring by regulators, open-access publication of safety incidents, and cooperation between companies and public health agencies will help fill these gaps. If future research exposes more significant hazards, swift policy updates and stronger labeling requirements should follow. Until more evidence emerges, common-sense lab precautions offer the most reliable shield.