History shows that some chemical discoveries reshape whole industries. 2-Methyl-1,3-propanediol (abbreviated as MPMD or MPDiol) fits this story. Since chemists first explored the field of diols in the mid-1900s, they started searching for molecules offering better performance, especially in plastics, coatings, and resins. MPMD’s structure—where a methyl group branches off the backbone—turned out to be more than a molecular quirk. By the late twentieth century, methods allowed for higher purity and bigger batches. Producers in the 1980s made it a standard raw material for polyester and polyurethane producers tired of the limits in other diols. Looking back, MPMD’s rise tracked shifting demands for tougher, more flexible materials.
MPMD, or beta-methyl-1,3-propanediol, earns its spot in production pipelines thanks to a simple but powerful formula (C4H10O2). The molecule has two hydroxyl groups, which means it slots perfectly into reactions that build long chains—ideal for polymer makers. With a clear, colorless liquid appearance, it brings a faint sweet smell, hinting at its chemical kinship with other glycols. This isn’t just a wet-chemistry curiosity; those who have run pilot batches of resins or adhesives often praise how well this diol blends and hardens in real manufacturing conditions.
Pick up a data sheet for MPMD and a few properties leap out. The substance melts around -54°C and boils just shy of 210°C, so it handles freezing cold and fairly high heat without changing form. In water, it dissolves well, surprising some who expect a branched structure to resist solubility. Density floats near 1.02 g/cm3 at room temperature—a tad heavier than water. Viscosity trends a bit higher than standard glycols, so pumping equipment needs to account for that. The methyl branch boosts chemical stability, which is a big reason why industrial processors pick it for tough applications. From my experience testing resins, I’ve found the hydroxy groups give plenty of sites for reactions, but the methyl group noticeably affects curing rates when you dial up the temperature.
Visit any chemical supplier with a focus on quality, and their labeling for MPMD reflects an emphasis on purity, typically 98% or higher for major applications. Material safety data sheets often flag it under the synonym Dimethylolpropane (DMP) or 2-methyltrimethylene glycol. This field expects tight control on moisture, color, and acid value. Those running large-scale productions usually set narrow specs: low water, minimal color (below 20 APHA), and almost zero metal impurities. Labeling needs to spell out batch numbers, storage advice, safety warnings, and—if exported—both local and internationally recognized product codes. This approach builds trust, and anyone shipping chemicals globally knows regulators watch every detail.
Chemists produce MPMD mainly by hydroformylating allyl alcohol, followed by hydrogenation. The process combines science with plenty of engineering, especially when reactor beds clog or yields slip. Companies chase yield and purity with elaborate purification steps—distillation columns tuned for the close boiling points of potential contaminants. Catalyst choice matters: rhodium-based systems offer selectivity, but not every plant wants to handle the cost or toxicity. Some R&D teams have been exploring bio-based routes, which would shift feedstocks away from oil and towards sugars or glycerol, but this approach keeps running into issues of scalability and purity. Talking to engineers in the field, it’s clear the hydroformylation/hydrogenation path is the workhorse, but rising pressures for ‘greener’ chemicals means alternatives keep getting attention in corporate R&D labs.
Try using MPMD as a diol in polyester or polyurethane syntheses and you quickly see what sets it apart from straight-chain glycols. The methyl group makes branching happen in the resulting polymer chains. These branches give finished products greater flexibility or impact resistance. I’ve seen coatings and foams with MPMD that shrug off dents instead of cracking. The two hydroxyl groups let it react with acids to form esters or pick up isocyanates in polyurethane processes. Crosslinking options open up, so chemists and engineers can thicken or strengthen products as factory needs shift. If you run experiments on side reactions, you’ll notice MPMD resists oxidation better than unbranched diols. Its resilience isn’t just textbook theory; in formulations on my bench, I’ve had fewer issues with degradation, even when I stretched process temperatures.
Walk through a warehouse or check vendor lists, and you’ll see MPMD go by many names. Two common ones show up the most: Dimethylolpropane (DMP) and beta-methyl-1,3-propanediol. Some catalogs list it under 2-methyl-1,3-dihydroxypropane or 2-methyltrimethylene glycol. European suppliers sometimes favor the DMP label, but US-based firms stick to MPMD or simply ‘Methyldiol’ in shorthand. For those mixing custom batches, clarity matters—especially when regulations set limits on naming to avoid mix-ups with similar glycols like neopentyl glycol or trimethylolpropane.
Anyone storing or using MPMD needs to pay attention to workplace safety rules. Inhalation of vapor is low-risk, but splashing or prolonged skin contact can irritate. I always recommend wearing tight-fitting safety goggles, gloves made from nitrile, and lab coats if you’re pumping or pouring large volumes. Spills get sticky, and the material’s viscosity slows cleanup. Storage tanks should keep out moisture and sunlight—hydroxyl groups grab water fast, causing issues in downstream processes. Fire hazard stays low, but don’t let open flames nearby. Waste disposal has to follow local environmental laws, with most sites funneling unused material for solvent recovery or incineration. Training workers to recognize labels, handle containers, and run emergency showers at the right moment has kept accident numbers low in factories where I’ve consulted.
MPMD’s qualities make it a favorite for companies turning out high-performance plastics, coatings, polyesters, and polyurethanes. Car parts built with MPMD-enhanced polyesters resist cracks during tough winter drives. Furniture companies rely on its branching for foams that don’t collapse under long use. In coatings, its chemical profile helps withstand scrapes—especially on industrial floors and heavy machinery. Polyester resins cured with MPMD show resistance to heat aging and UV, a valuable trait in paints for bridges or stadium seating. Fiber industries, always hunting for smoother spinning and greater elasticity, add MPMD for better textile performance. Latex adhesives, once plagued by brittleness, gain durability from its branching structure. Years spent helping formulators troubleshoot failures have shown me that MPMD isn’t just about technical advantages on paper—it consistently solves wear, flexibility, and resilience gaps in real-world products.
Researchers have pushed both synthesis and applications for MPMD, always hoping to leap past existing limits. Labs aim to drop fossil-derived feedstocks and explore renewable production, like fermenting sugars into precursor molecules. Small batches have reached high purity, but scaling remains tough—contamination, cost, and reproducibility slow progress. Some chemists experiment with new catalysts to cut waste and energy use. On the application side, recent patents describe specialty polyurethanes for medical devices and electronics, where MPMD plays a role making materials biocompatible and tough without brittleness. Industrial teams keep probing for MPMD’s edge in reducing VOCs in paints and coatings. Having sat in on brainstorming sessions, I see optimism balanced by the challenge of matching cost and performance with existing materials. Data from collaborative R&D points to hybrid materials—blends that mix MPMD with other diols—emerging as a focus, promising greater balance between impact resistance and weathering.
Numerous toxicity studies reassure but remind us not to get complacent. MPMD has shown low acute toxicity in studies with rats and rabbits, with high oral LD50 values and mild irritation as the main concern from skin contact or inhalation of concentrated vapor. Long-term studies haven’t flagged significant carcinogenic or reproductive risks, though ongoing monitoring remains the smart path. Aquatic studies highlight fast biodegradation, and it doesn’t readily bioaccumulate, lowering risk to waterways if minor spills occur. Still, handling protocols stress the importance of engineering controls, especially for air quality in tight spaces. I pay close attention to updates on European REACH and US TSCA compliance, because regulatory winds shift fast when new data emerges. The story of phthalates and similar chemicals stands as a reminder to never assume a chemical’s safety profile is final.
Growing demand for tougher, lighter, and more sustainable materials points toward a bright future for MPMD in manufacturing. Efforts to cut carbon footprint will keep researchers searching for bio-based routes and cleaner production. As companies invest in circular economy models, MPMD’s recyclability within polymer matrices emerges as a selling point. More consumer brands ask suppliers about chemical origins, pushing transparency and supply chain verification. Blends with other specialty glycols may unlock new coatings, fibers, and foams that outperform traditional products but meet eco-certification standards. Continued success will depend on balancing cost, safety, and innovation—a lesson from every phase in the life of this versatile chemical.
Most folks don’t realize how many modern products depend on specialty chemicals like 2-Methyl-1,3-Propanediol, usually called Methylpropanediol or MPG. Even if you’re not in the lab, you interact with this stuff more than you think. MPG serves as a building block—think of it as Lego pieces for large chemical structures. Factories use it for all sorts of reactions that lead to things we touch and use daily.
MPG finds a big role in making polyesters. These aren’t just for fabrics. Polyesters go into making films for packaging, coatings for metals, and bottles for soft drinks. The structure of MPG helps give polyesters a tough, flexible nature. I remember touring a packaging plant a few years ago; engineers pointed out that tiny tweaks in the chemical structure can keep a soda bottle strong but lightweight. That tweak often comes from MPG. Without it, you’d get more bottle breakage on the way to the grocery store, and the material wouldn’t recycle as smoothly.
MPG isn’t just another petrochemical. Engineers look at it for greener, bio-based solutions, especially when making high-performance fibers. Think about moisture-wicking shirts or stain-resistant upholstery. MPG helps create fibers that are comfortable, durable, and easier to clean. It stands out because it resists breaking down in sunlight, which makes clothes and furnishings last longer, ultimately saving money and reducing waste.
Polyurethane wouldn’t be where it is today without reliable diols like MPG. If you sit on a couch with soft, bouncy foam or walk in a pair of comfy sneakers, you’re feeling the benefits. MPG lets manufacturers tailor softness, density, and toughness without extra steps or extra chemicals. Rapid-curing adhesives and coatings owe a lot to this compound, giving rise to safer assembly lines and less downtime.
MPG shows up in specialty fluids for automotive and electronics applications. Coolant fluids that protect computer chips or car batteries rely on stable molecules like MPG. It fights off corrosion, resists freezing, and has a safety profile that’s a step ahead of older glycols. The versatility means less downtime due to equipment failure—a huge plus for big data centers or electric vehicle fleets. In my own work with electronics suppliers, fluid breakdown often caused surprise outages, and more stable chemicals like MPG always cut those incidents.
Cosmetics and personal care companies appreciate how MPG dissolves ingredients smoothly and doesn’t irritate the skin. Hand lotions, shampoos, and even toothpaste list MPG as an ingredient that helps blend moisturizing oils with water-based ingredients. Compared to some classic chemical alternatives, MPG rarely triggers allergic reactions, which explains why dermatologists started recommending MPG-based formulas to patients with sensitive skin.
MPG benefits from a track record for safety and reliability, but growth in demand brings pressure to keep sourcing responsible. Producers have started shifting toward renewable feedstocks so that the next generation of MPG-based products leave a smaller carbon footprint. Recycling networks, greener chemistry methods, and new quality standards can help industries keep pace while meeting environmental expectations.
Working in a manufacturing setting, names like 2-Methyl-1,3-Propanediol crop up more than most folks realize. This diol sits in polyester production, eco-friendly plastics, and paints. It can soften resins or act as a base ingredient for many day-to-day items, so it genuinely touches a lot of both industry and regular homes. People ask if it’s safe, and the answer is layered.
Direct skin contact with 2-Methyl-1,3-Propanediol doesn’t burn or blister right away. It lacks the volatility or sharp scent that makes other industrial chemicals an instant warning. Breathing in its fumes isn’t likely in a regular ventilated space. Still, skin irritation isn’t rare, and swallowing it becomes a whole other risk — one that could require a call to a poison center. Safety data from the European Chemicals Agency and American organizations confirms: gloves and eye protection matter during every shift.
In my own experience running a small plastics shop, forgetting gloves once meant my hands felt gross and tight halfway through the day. A coworker got some in his eye; the watery stinging look on his face told us enough. We rinsed for twenty minutes and called a supervisor. Water, lots of it, usually handles minor exposure. But medical attention sometimes makes sense for bigger spills, splashes, or accidental drinking (kids' curiosity or distracted workers).
One reason 2-Methyl-1,3-Propanediol interests researchers involves its lower toxicity compared to many other glycols. Many see it as a greener choice. It still doesn’t belong near kids’ playgrounds or in open fields. The chemical breaks down over time, but not fast enough to ignore spills or leaks. No one wants hidden runoff winding up in a well or drinking supply.
People sometimes think “greener” or “less toxic” means safe for long-term skin or lung contact. Real life says even less toxic substances contribute to air and water pollution if policies go ignored. Workers should expect clear training, and management should provide strong ventilation as standard.
Good industrial practice looks like labeled containers, gloves within arm’s reach, and easily read instruction sheets. I’ve seen new hires given hand-me-down aprons and safety goggles with smudged lenses—no good. Investing in the basics pays off in fewer sick days and fewer trip-ups with regulators.
Even small businesses can build cultures around routine safety checks. A checklist on every doorframe, fresh reminder signs above every sink, simple spill response kits — these moves get taken for granted until the one day they make all the difference.
Many companies have started using less hazardous ingredients in part because customers and local officials demand stronger oversight. People outside the factory walls have voices, too. If you run a school or maintain public facilities, demand clear Material Safety Data Sheets on every shipment. If you’re at the end of the line, using finished products, keep labels and instructions nearby. The safest route will always be: treat every unfamiliar chemical with a little healthy suspicion, keep protective gear handy, and never shortcut clean-up and storage.
Experience says most mishaps start with small lapses — a forgotten glove, a torn label. Put effort into the basics; the rest falls in place.
Long chemical names often look intimidating, but it gets easier if you break them down. Take 2-Methyl-1,3-Propanediol as an example. The “propanediol” part tells you this molecule has three carbon atoms and two alcohol (-OH) groups. “1,3” signals those -OH groups attach to the first and third carbon. “2-Methyl” flags a single carbon sticking off the middle carbon. In daily research, I find this style of naming tells a fairly simple story if you pick it apart step by step.
This compound pops up in the world of organic chemistry because of its unique structure. It packs two functional alcohol groups, which means it can react at two separate locations. In a lab or manufacturing setting, this dual function comes in handy for making polymers or specialty solvents. My background in synthetic chemistry reminds me how often structures like these come up in real-world material design.
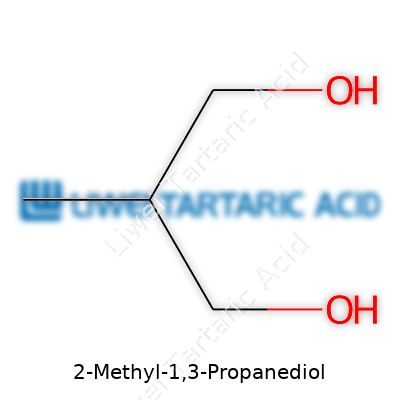
The straightforward chemical formula for 2-Methyl-1,3-Propanediol is C4H10O2. Count the atoms: you get four carbons, ten hydrogens, and two oxygens from those two -OH groups.
If you draw it out, the main backbone forms like this: three carbons in a row, with -OH groups at either end. That extra methyl group pops off the middle carbon. The condensed structure comes to: CH3-CH(CH3)-CH2OH. Seeing this, you know the molecule branches out, unlike plain 1,3-propanediol, which stays linear.
Visual representation matters, especially for people starting out in chemistry. I remember students handling models and suddenly understanding why the extra methyl group changes molecular symmetry and reactivity. This small tweak alters how the molecule fits into polymer chains or interacts with enzymes if you use it in biotechnology.
2-Methyl-1,3-Propanediol has found a place in several industries. Its branched molecular skeleton makes it more resistant to crystallization, letting products like resins and polyurethane foam stay flexible in cooler temperatures. Few people notice that tiny structure shifts—like the presence of a methyl group—can make coatings tougher, or plastics feel softer, with less shrinkage over time.
Chemical suppliers cite 2-Methyl-1,3-Propanediol as an alternative to regular glycols when a formula needs better chemical stability or biodegradability. I’ve seen environmental research point out how this compound breaks down more easily in soil and water compared to some diols out there, so it fits well with stricter regulatory rules.
Anyone curious about chemical safety will appreciate that this diol typically rates lower on the toxicity spectrum. Still, it pays to follow best practices in the lab or factory—use gloves and keep ventilation strong, since alcohols can still irritate the skin and airways.
Scaling up greener production of 2-Methyl-1,3-Propanediol calls for innovation. Classic production relies on starting materials from petroleum. Some companies are switching to biobased routes, using fermentation or catalysis from renewable feedstocks. Research from recent years backs this up—biosynthetic pathways deliver similar product quality, and early life cycle analyses point to smaller carbon footprints.
Looking ahead, a better grip on the physical and chemical quirks of 2-Methyl-1,3-Propanediol could unlock fresh uses in advanced manufacturing or sustainable consumer goods. Small steps in chemical design, like moving a methyl group, wind up shaping industries in ways most of us don’t expect.
Anyone who has ever handled a drum of chemicals understands how important it is to avoid simple mistakes. With 2-Methyl-1,3-Propanediol, it’s easy to think, “Just another clear liquid,” but skipping the details turns into wasted product or even health hazards. Though not a household name, this chemical finds its way into paints, coatings, and cosmetics—products that people use every day.
Sealing containers tightly always tops my list. Once, I watched a small leak leave a sticky mess on a warehouse floor. Not only did the team spend hours cleaning, that spill could have caused someone to slip or ended up in a drain. A closed container stops contamination from dust and moisture. It also prevents vapors from sneaking into the air. That seems basic, but I’ve seen experienced teams overlook it.
I always check for a cool, dry spot far from sunlight or any hot surface. High temperatures don’t just degrade the product—a little heat in a poorly ventilated room can increase pressure inside the drum. Every storage guide for glycol derivatives repeats this advice, and for good reason. I once saw a batch ruined when it sat near a window during summer. The liquid started to yellow, and the company lost a chunk of money.
Keep 2-Methyl-1,3-Propanediol away from acids and strong oxidizers. Decades spent in storerooms have taught me that mixing the wrong materials can cause nasty reactions. Even if the risk looks low, isolation keeps accidents small and manageable. Storing this chemical on dedicated shelving adds peace of mind, and nobody has to wonder if leftover acid from a spilled cleaning solution will start something dangerous.
Ventilated storage areas should never feel optional. The scent in a stuffy storeroom tells its own story—vapors build up quickly, especially where airflow doesn’t reach. Not only can this increase exposure risk for workers, but some glycol vapors might trigger alarms or irritate nearby staff who aren’t wearing protection. OSHA guidelines recommend this as a best practice, and the extra fan goes a long way for everyone’s comfort and safety.
Every pail deserves a clear, unambiguous label. Relying on memory or makeshift tags leads to confusion, especially as stocks turn over. All relevant hazard codes and storage instructions should be front and center. I remember a time when a faded label caused a delay as the team double-checked compatibility for a disposal run. The right sticker saves time and mistakes.
PPE should always be on hand: gloves, goggles, and a long-sleeved coat. A splash might seem unlikely, but one drop in an eye lands a person in the ER for hours. Eye-wash and spill kits turned up close to storage racks limit the fallout and help teams act fast. Practicing with these tools matters—a group that never runs a drill ends up scrambling in the real thing.
Safe storage protects products, workers, and reputations. Companies caught mishandling chemicals face more than fines. Customers want peace of mind, and so do employees. Every detail, from airtight seals to safety drills, forms a chain that supports daily operations. In my experience, teams that care about these steps earn trust from both regulators and clients, building a safer working world.
2-Methyl-1,3-propanediol, often called MPDiol, shows up in all sorts of places—coatings, fibers, and even cosmetics. Its clear and nearly colorless liquid state tells you a lot straight away. A chemical doesn't have to shout to prove valuable; its consistency in form and behavior pulls its weight in production lines. The viscosity stands out here—not too thick, not too watery, making it easy for pumps and pipelines to handle. Its mild aroma doesn't overpower a workspace or finished product. That helps when companies aim for clean, neutral scents or want to avoid introducing odors into their goods.
MPDiol only begins to solidify at low temperatures, with a melting point dropping just under room temperature. You rarely see it freeze up in storage or transit, even in cold climates. The boiling point sits high, above 200°C. Heat it, and it holds together well before vaporizing. In my experience, that high boiling point relieves headaches for anyone needing stability during hot reaction steps. You get more leeway and less worry about losses through evaporation mid-process.
Water and MPDiol blend easily. Try mixing it with solvents like ethanol or acetone, and you'll find the same result. This feature turns it into a team player in many applications. Coating manufacturers, for example, appreciate solubility that simplifies the blending of raw materials. No fuss, no waiting for hours trying to get a homogenous batch. From a worker’s angle, nothing ruins a day faster than stubborn solids in a tank.
This glycol derivative feels denser than water, clocking in at about 1.02 to 1.04 grams per cubic centimeter. In practice, you pour it and notice immediately how it doesn’t splash like water, but doesn’t slump heavily like honey. This makes container filling less prone to spillage. Storage tanks, transport drums, and shop-floor operations all benefit from its stable and predictable pour.
Since MPDiol carries two hydroxyl groups, it offers plenty of hooks for chemical reactions. Polyester and polyurethane producers lean on these groups to build tough, durable materials. This reactivity, though, means you need to keep it away from strong oxidizing agents. It won’t explode at random, but caution and proper labeling save costs and prevent mishaps in the warehouse.
Producers notice that MPDiol handles exposure to air and moisture better than some older glycols. It doesn’t absorb water as aggressively, which stops it from going sticky or slushy over time. That makes a difference for small startups without expensive climate control in storage spaces. Less worry about product breakdown or waste saves both time and money.
MPDiol avoids many of the toxicity issues seen with some glycols used decades back. Most safety data point to minimal skin and inhalation hazards, as long as workers follow basic hygiene and wear gloves if contact risk runs high. Forced ventilation in large-scale operations keeps exposure even lower. Personally, I look for chemicals that allow a margin for error; people make mistakes, and safer choices keep teams healthy and businesses stable.
Customers want materials that check the right boxes for safety and environmental impact. Thanks to its low volatility and non-hazardous waste profile, MPDiol holds a spot on modern green chemistry lists. Responsible disposal, regular audits, and spill prevention plans round out the strategy. Experience tells me that upfront responsibility beats cleanup every time.