The story of 2-Chloro-1,3-propanediol stretches back to the mid-20th century, growing alongside the food and chemical manufacturing sectors. Chemists discovered that this compound sprang up during processing and refining, especially in foods cooked at high temperatures or with acid-hydrolyzed vegetable protein. The rush to industrialize food production brought it into commercial spaces, even before folks understood all its side effects. By the late 1990s, with regulators and scientists paying more attention, 2-Chloro-1,3-propanediol started drawing headlines due to concerns over toxicity, prompting closer scrutiny and updated standards for food safety tests.
2-Chloro-1,3-propanediol, often called 3-MCPD, crops up in processes involving fat and protein hydrolysis. Manufacturers use related compounds or discover them as byproducts in everything from soy sauces to paperboard packaging. Industry codes and trade groups list dozens of variations and grades. It shows up not just in processed foods, but also in certain surfactants and resins, a reminder of how chemical advances sometimes outrun their regulation.
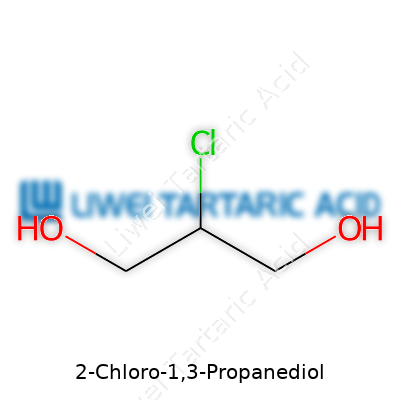
Holding a clear to pale yellow appearance, 3-MCPD comes across as a slightly viscous liquid that's easy to dissolve in water and other polar solvents. Its molecular formula is C3H7ClO2, and it weighs in at roughly 110.5 g/mol. It boils just above normal water temperatures, which means handling during industrial reactions calls for real care. It starts to break down when exposed to strong alkalis or heat, releasing potentially hazardous byproducts, and gives off a sharp, medicinal odor.
In most settings, suppliers must detail purity levels well over 95%, and list allowed levels of related contaminants, like glycidol or other chloropropanols. Packaging includes hazard warnings, standard GHS labeling with clear signifiers, because regulations require explicit information on exposure limits and handling precautions. Shipment documentation usually spells out flash points, melting points, and storage instructions, so downstream users get a clear sense of safety needs.
3-MCPD comes from heating fats or oils in the presence of hydrochloric acid or other chlorinated reagents. This triggers a reaction that splits off fatty acids and leaves behind chlorinated glycerol derivatives. The process has roots in soap manufacturing and food seasoning production. Industrial methods rely on tight temperature control and quick separation, because side reactions easily kick in at just slightly higher temperatures, spawning unwanted contaminants.
Once produced, 2-Chloro-1,3-propanediol can undergo further changes. In alkaline environments, it hydrolyzes quickly, turning into glycerol and various chlorohydrin species. Chemists sometimes convert it into surfactants or epoxides for specialty plastics and resins. Its reactive hydroxyl and chlorine groups let researchers test new chemical routes to plasticizers, solvents, and specialty intermediates, but these modifications demand real attention to safety because of the compound’s toxic risks.
3-MCPD goes by plenty of other handles, depending on the industry and country. Common names include 1,3-dihydroxy-2-chloropropane, α-chlorohydrin, and glycerol α-monochlorohydrin. These synonyms pop up in regulatory filings, on ingredient lists in processed foods, or across chemical supplier catalogs, making it tricky for users and regulators to keep track. Anyone working with this compound needs a careful checklist to trace all its forms and possible hidden sources.
Governments have stepped in, setting tough exposure limits for 3-MCPD in food and workplaces. The World Health Organization capped tolerable daily intake at roughly 2 micrograms per kilogram of body weight. Europe, China, and the U.S. enforce maximum allowable concentrations in everything from sauces to baby formula. Standard operational practice means using gloves, goggles, and fume hoods, plus rigorous regular air monitoring in plants. If a spill occurs, workers quarantine the area and use special absorbents to avoid inhalation or skin exposure, since it’s considered a possible carcinogen.
Most people encounter 3-MCPD through processed foods, especially Asian-style sauces, which often use acid-hydrolyzed vegetable protein. Less visibly, it lurks in some paper or cardboard coatings and in minor quantities in industrial surfactants, soaps, and resins. Almost every regulatory incident or contamination scare involving 3-MCPD ties back to unexpected byproducts in manufacturing, usually due to shortcuts or outdated cleaning protocols.
Researchers push for better tools to track and eliminate 3-MCPD and its related contaminants. Innovations in gas chromatography and mass spectrometry have made it easier and quicker to spot even trace contamination, leading to stricter recalls and reformulations. Seed companies chase plant oils and protein crops that naturally avoid high MCPD formation. Technology firms look for ways to swap out acid treatments or redesign process steps to prevent formation in the first place, motivated by both consumer pressure and sharp legal penalties in major food markets.
Toxicological studies have produced real concern. Lab experiments in rodents link 3-MCPD to kidney and testicular tumors, decreased fertility, and changes in blood chemistry. Evidence triggered limits on food and water exposure decades ago. While direct links to similar risks in humans remain uncertain, regulators take a precautionary stance, especially for babies, young children, and pregnant women, who may face the highest risks from exposure.
Companies face a clear fork in the road: keep defending the status quo, or invest in cleaner and greener techniques. Expect stricter regulations and regular downward revisions of allowed concentrations in foods and packaging. Labs and manufacturers need to move faster on adopting safe substitutes, optimizing process conditions, and overhauling QC screening. Public tastes—driven by both science and skepticism—are turning away from compounds flagged as risky, so suppliers can't count on current practices holding up much longer. Newer plant breeding and green chemistry promise some hope, but the industry’s track record shows that only real regulatory teeth force serious cleanup.
2-Chloro-1,3-Propanediol, which sometimes shows up in the food industry’s list of concerns, touches more lives than you’d think. It isn’t a household name, yet it’s often woven into debates around food safety and industrial processing. Some may recognize it by its shorter name, 3-MCPD. It usually pops up as a contaminant in processed foods, especially those containing hydrolyzed vegetable proteins and some soy sauces.
Public health experts started tracking 3-MCPD levels after discovering it during investigations into soya product production. During acid hydrolysis, the process used to break down plant proteins, 3-MCPD can form. This raised alarms because certain studies carried out on lab animals found a link between this compound and kidney damage, as well as cancer risks with high, long-term exposure. Understandably, this led to serious discussions about regulation and limits.
Industrial production often brings these kinds of complexities. Many companies wanted to speed up protein breakdown to mass-produce savory seasonings and taste enhancers. Acid hydrolysis makes the process efficient, but it opens the door for 3-MCPD to form if temperatures and processing methods aren’t closely monitored. To protect health, food safety agencies in Europe, North America, and parts of Asia agreed on established limits for acceptable levels in foods, keeping daily exposure below thresholds shown to stress animal kidneys in research studies.
As someone who keeps an eye on shopping labels, I noticed more soy sauces now claim to be “3-MCPD free” or “naturally brewed.” This isn’t a marketing gimmick. Companies using older, chemical-heavy processes are under pressure, because consumer trust relies on safety assurances. In labs, analysts use high-tech methods like gas chromatography to detect even trace amounts. Producers switching to natural fermentation processes reduce risk, but these methods take longer and cost more. Choosing safe, slow-brewed options stands out as the smarter call, both for peace of mind and long-term health.
Food isn’t the only place 2-Chloro-1,3-Propanediol appears. Some manufacturers use it as a building block in producing other chemicals, including certain resins and lubricants. Here, strict safety protocols limit worker exposure and environmental releases. Still, it’s the food link that hits closest to home, since public health risks often turn up in the foods people eat every day. Research keeps evolving on the long-term effects of consumption, especially since byproducts can end up in cooking oils and baby foods if production isn’t scrubbed clean.
The responsibility sits between regulators, food producers, and watchdog scientists. Regulations on 3-MCPD in food continue to tighten. The European Food Safety Authority slashed acceptable intake limits, pushing companies to invest in better technology and fermentation know-how. Pressure mounts on industry leaders to transition from fast, chemical-based shortcuts to safer, time-tested brewing techniques. As more consumers demand transparent sourcing and clear labeling, companies lagging behind risk losing market share. Building trust means sticking with best practices, regular batch testing, and a willingness to adapt processes as science uncovers new evidence.
By focusing on up-to-date technology, better factory oversight, and regular public reporting, food safety can move in a positive direction. Changing old habits takes commitment, but the payoff is a food system where risks from compounds like 2-Chloro-1,3-Propanediol stay firmly in check.
Not everyone comes across the name 2-Chloro-1,3-Propanediol in daily life, but many have unknowingly eaten foods that contain very small amounts of it. Most folks in the food and chemical industry call it 3-MCPD. This compound pops up in certain processed foods, soy sauces, and flavorings when fats or oils get exposed to high heat with chloride ions around. If you’ve ever wondered why food safety experts keep a close eye on some sauces, here’s one reason why.
Scientists have studied 2-Chloro-1,3-Propanediol for decades because it doesn’t just slip through the body without leaving an impression. Tests with lab animals point to potential kidney damage and changes in their reproductive system after high, repeated exposure. The World Health Organization recognized these risks, setting limits on just how much should appear in food. Europe follows with strict levels allowed in food products containing vegetable oils and their derivatives, leaning on animal research as a guide for public health protection.
Years working in quality assurance for a food manufacturer opened my eyes to the challenge. Analysis of products sometimes showed trace amounts of 3-MCPD in hydrolyzed vegetable protein, especially where acid hydrolysis gets used. We learned early on to monitor batches, adjust processing conditions, and substitute safer methods so we didn’t trigger recalls or damage consumer trust. Government labs back up this strategy—setting tough standards, running checks on imports, and keeping producers on their toes.
This isn’t just an academic debate. High doses in lab experiments link to tumor formation in rodents, prompting some agencies to label 2-Chloro-1,3-Propanediol as a probable carcinogen. The amounts found in food are way below these levels, but long-term, steady exposure still stirs up worry. My own approach at the dinner table has changed; I look at ingredient lists closely, especially for young children, since their smaller bodies handle chemicals differently.
Simple solutions can make a difference. Manufacturers have switched to gentler processes and tighter temperature control to keep levels low. Some retailers now demand stricter testing from suppliers, holding brands accountable for what ends up on store shelves. As a parent, I appreciate these efforts. Awareness isn’t just for food scientists or government regulators. It becomes a habit—choosing products with transparent labeling, reaching for naturally brewed soy sauces, or supporting brands that test for 3-MCPD.
Talking about food safety sometimes stirs up needless panic, but real risks deserve careful attention. 2-Chloro-1,3-Propanediol reminds us that behind every plate of processed food, a whole chain of choices works to keep it safe. Progress comes step by step, with honest research and public standards rooted in health, not fear. Being mindful, pressing for transparency, and listening to experts turns attention from worry to prevention—and that’s something seen every time you visit the grocery store.
2-Chloro-1,3-propanediol turns up in conversations about food safety, mostly because it goes by the name 3-MCPD in scientific circles. Its chemical formula is C3H7ClO2. The structure packs three carbon atoms, seven hydrogens, one chlorine, and two oxygens. This arrangement isn’t just a trivial piece of trivia. It serves as the backbone of how the molecule interacts with living systems and ends up drawing attention from food agencies worldwide.
Most folks encounter traces of 2-chloro-1,3-propanediol in processed foods, especially in sauces, oils, and baked products. That’s because it often forms during high-temperature processing, such as refining vegetable fats or making soy sauce. The presence of chlorine in this compound’s structure means it’s chemically reactive and not something the body naturally expects. Scientists at the European Food Safety Authority flagged concerns as early as 2016, referring to evidence from animal studies where exposure triggered a cascade of health effects, including kidney damage and changes in male fertility.
The specific arrangement in C3H7ClO2 underlines how easily 2-chloro-1,3-propanediol slips into our food chain. Every corner store or supermarket has items where residues might show up beneath the radar. I’ve looked at food labels and heard friends talk about snacks; deep down, nobody expects a chemical with chlorine tangled into its structure to ride alongside familiar ingredients, but that’s the reality.
Parents, students, and cooks ought to recognize that awareness begins with understanding what’s in our food. The food industry, for its part, can’t always prevent 3-MCPD formation, but processes can be tweaked to keep levels low. Scrutiny from watchdog groups matters because research suggests even low-level contamination, over time, could add up.
Tackling this issue doesn’t fall on a single person’s plate. Research teams across Europe and Asia have more or less shown that a switch toward indirect heating in oil refining, using lower temperatures, can trim down the rate at which 2-chloro-1,3-propanediol forms. Soy sauce manufacturers have started to apply enzyme treatments and tweak their fermentation times so that the levels of this unwanted byproduct don’t climb too high.
Regulators have also set strict limits to protect consumers. The European Union capped the maximum allowed content in infant formula and vegetable oils, which forced producers to invest in better lab testing technology. Some rapid tests can now spot residues at the parts-per-billion level—barely a whiff, but enough to keep food safe.
Drawing from real concern and personal habit, reading food safety alerts and keeping an eye on regulatory reports brings peace of mind. No system works perfectly, but the more consumers, scientists, and regulators talk and share information about what chemical formulas like C3H7ClO2 really mean, the better our odds at cutting invisible risks. The path to safer food rides on clear facts, shared vigilance, and a willingness to improve food technology for healthier futures.
Anyone who’s ever worked in a lab knows accidents tend to happen when people get casual about chemical storage. I’ve seen leaky bottles take out a bench’s worth of tools overnight, and that’s nothing compared to the risks of improper storage for something like 2-Chloro-1,3-Propanediol. This chemical brings clear hazards, both for health and for the environment. Its toxic potential isn’t hypothetical. Long story short, direct exposure can irritate skin and eyes, and inhaling or ingesting even small amounts can spell trouble.
Early on, I learned to look past the paperwork and focus on daily practices. Keep this compound in a tightly sealed container made from glass or resistant plastic—polyethylene or polypropylene stand up well. Skip the standard shelving if possible and use a dedicated area away from acids, oxidizers, and bases. This chemical breaks down if exposed to sunlight or extreme temperatures, so a cool, dry, well-ventilated cabinet works best.
Humidity creeps in faster than you’d think, and given 2-Chloro-1,3-Propanediol’s tendency to absorb moisture, I always toss in a desiccant pack. It makes cleanup simpler if something does spill, and limits product breakdown. Store everything at room temperature, far from heat sources, ovens, or sunlight streaming through a window. Labeling often gets overlooked, but it saves more than just time. A missing or smudged label can turn a minor moment into a real emergency, so always write the chemical’s full name, concentration, and hazard class right on the bottle.
I’ve worked in busy labs where people rush through their routines. Splashes, drips, and cross-contamination happen in seconds. Basic gloves do little for chemicals like this—go for nitrile or neoprene gloves, not latex. Goggles and a face shield cut the risk if a splash jumps toward your face. Always use a fume hood. This keeps the vapors away from your nose and mouth, and protects everyone else nearby too.
Pouring or measuring should stay on a tray with raised edges, just in case. I watch for nearby waste containers and never reuse pipettes or spoons between chemicals. Those shortcuts sound faster, but create new hazards. Never return unused material to the main bottle—contamination builds, and a single mistake can affect every batch that follows. Instead, dispose of leftovers in a clearly marked solvent waste container, not down a sink or drain.
Learning from colleagues’ mistakes, I’ve seen the fallout from skipped steps. As soon as possible, wipe up spills with absorbent pads, working from the edges inward. If skin contact happens, flush immediately with lots of water and take it seriously—even small stings mean you’ve been exposed. Report any accident, no matter how minor it feels. Small problems can snowball fast, especially with chemicals like this.
Staying sharp, labeling bottles, double-checking gloves, and staying in the routine of using a hood become second nature with time. People talk a lot about best practices, but real safety comes from habits picked up day to day. Sharing this know-how, and learning from years on the job, helps everyone avoid careless mistakes and keeps a demanding work environment safer for all. If I’m teaching a new lab member, I stress vigilance. Over-familiarity with hazardous chemicals has no upside, but careful storage and handling pays off every day.
Sitting down to write about 2-Chloro-1,3-propanediol, I keep thinking about the food safety recalls you hear about every couple of months. This compound, called 3-MCPD for short, shows up when processing fats and proteins in foods at high temperatures, especially during the production of soy sauce, margarine, and some baked goods. I remember standing in a grocery aisle, scanning labels for anything suspicious after news broke about contaminants in some processed foods. Food manufacturers face real headaches because of the microscopic levels of 3-MCPD that can develop while refining oils or hydrolyzing vegetable proteins. Regulators in Europe and Asia watch it closely, setting strict limits and nudging companies to upgrade their processes to keep this contaminant in check. While it comes up as a byproduct, attention to this issue protects public health and forces the whole industry to pay closer attention to chemical transformations in recipes and machinery.
I spent a few years working at a chemical plant, and 2-Chloro-1,3-propanediol got flagged on more than one safety sheet. Producers of specialty chemicals prize this molecule as an intermediate—a building block for making things like pharmaceuticals, biocides, and surfactants. It winds up in the middle of reaction pathways because the chlorine atom gives chemists a good handle for modifying the molecule, attaching new functional groups or breaking it down further depending on the needs of a particular synthesis. In pharmaceutical research, everything from antiviral agents to hypertension medications sometimes passes through stages where this compound plays a supporting role. Its strong reactivity makes it valuable, but also means it demands careful handling and robust safety training for anyone working with it.
Factories making paper coatings and finishes use 2-Chloro-1,3-propanediol to produce certain resins. These resins add moisture resistance and durability to paper and cardboard. I had a friend who worked in pulp and paper, and he'd tell stories about the precision needed in dosing chemical additives. Too much, and you get sticky machinery and wasted inventory. Too little, and the product doesn’t last through shipping. In textiles, manufacturers put coatings onto fabrics that help them repel water or resist stains, often relying on derivatives produced from this molecule for performance.
The attention given to 2-Chloro-1,3-propanediol underscores a broader push by both regulators and industries to clean up production lines. In food, it means tweaking process conditions—like lowering reaction temperatures or switching up additives—to keep byproducts well below regulatory limits. Laboratories constantly look for less hazardous alternatives in chemical and pharmaceutical production; green chemistry approaches steer companies toward safer synthetic pathways. Paper and textile mills rely on closed-loop systems and improved ventilation to minimize worker exposure. All these steps, in their own way, highlight that industrial chemistry isn't just about efficiency. It’s about health, precision, and responsibility—not only to the final user but to everyone along the chain.
Consumers push for transparency and better labeling as they learn more about trace chemicals in food and household goods. Regulators continue research to clarify what levels are truly safe in the diet or work environment. Across every industry touched by 2-Chloro-1,3-propanediol, smart and careful design now guides product development. That attention not only protects current customers and workers—it shapes expectations for every generation that comes next.