The journey with 2-Amino-2-Methyl-1,3-Propanediol, often known as AMP or simply Aminomethylpropanediol, began nearly a century ago, carved out by necessity during the rapid advancement of organic chemistry. Chemists hunted for versatile building blocks in pharmaceuticals, coatings, and synthetics. The compound’s unique combination of amine and alcohol groups presented opportunities to tweak amino alcohol chemistry for buffer solutions in analytical chemistry and various medical uses. Documentation from the 1930s shows scientists in North America and Europe investigating its buffering capabilities, experimenting in labs before commercial synthesis scaled up in the 1950s. Over decades, the material became a staple ingredient for chemical manufacturers, research institutions, and pharmaceutical labs, laying groundwork for modern buffer systems and synthetic routes in drug design.
The compound presents itself as a colorless, sometimes faintly yellowish, crystalline solid, with a notable ability to attract moisture from air. That trait makes it convenient as a buffer in biological media, but requires airtight packaging to avoid clumping and water absorption. Industry standards produce it in a purity exceeding 99%, and supply it in kilolab scales for industrial use or in smaller bottles for laboratory work.
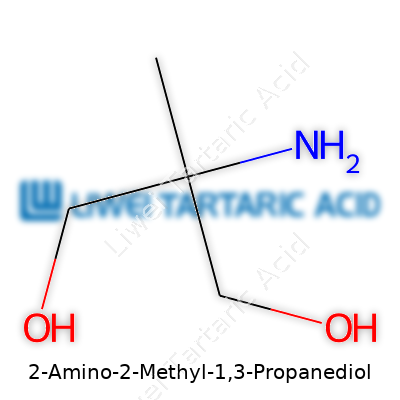
With a molecular formula of C4H11NO2 and a molar mass just above 105 grams per mole, AMP stands out due to its two hydroxyl groups and a primary amine, both attached to a propane backbone. The substance readily dissolves in water, contributing to its reputation as an effective neutralizer and buffer. Its melting point hovers around 48–52°C, and decomposition only occurs far above 200°C if exposed to high heat. The chemical acts as a mild base, with a pKa close to 8.8, giving it a sweet spot for controlling pH in cosmetic and pharmaceutical mixtures.
Industry labels call for straight language, highlighting purity percentage, batch or lot number, production date, and storage conditions. Specifications typically address water content, residue after ignition, color (measured via APHA units), and absorption characteristics via infrared spectroscopy. With increasing demand for traceability and sustainability, major suppliers offer full certificates of analysis, as well as data sheets laying out recommended shelf life and guidance for safe handling. Regulations in North America and Europe require clear hazard statements because—despite its reputation as relatively low toxicity—contact with eyes or prolonged skin exposure causes irritation.
Commercial synthesis generally starts from acetone and hydrogen cyanide, forming acetone cyanohydrin, which undergoes amination with ammonia and subsequent hydrolysis. Every facility modifies these steps, catering to available starting materials and energy use goals. Small-scale labs might reduce nitropropanediol through catalytic hydrogenation, though this method fits mostly for curiosity-driven research or rare isotopically labeled materials. Modern plants monitor each stage for trace impurities, recycling solvent and controlling waste streams to match environmental standards.
In chemistry labs, 2-Amino-2-Methyl-1,3-Propanediol behaves predictably thanks to its well-understood reactive groups. It forms salts with acids, enabling preparation of buffer systems. Condensation reactions with aldehydes or ketones offer routes to more complex molecules used in drug design or polymer development. Chemists employ derivatization on analytical instruments to measure trace amounts in mixtures. The diol functionality provides handles for ester formation or crosslinking in polymer matrices, which has relevance for coatings and adhesives engineering. As an intermediate, AMP supports the synthesis of beta-lactam antibiotics and acts as a platform for producing surfactants and emulsifiers.
While chemical language often confuses newcomers, 2-Amino-2-Methyl-1,3-Propanediol keeps recognizable aliases. The abbreviated AMP features across scientific articles and product catalogs. Suppliers and researchers also use the names aminomethylpropanediol and AMPD. Some labels reference the compound as "2-Amino-2-Methylpropane-1,3-diol" or, less commonly, as isopropanolamine. Marketing documents sometimes list it under trade names reflecting manufacturer branding, although the molecular structure remains the same across vendors.
Handling AMP in the lab or factory brings a straightforward routine. Safety data highlights the need for protective gloves, goggles, and access to an eyewash station, echoing habits drilled into chemistry students and seasoned workers alike. Inhalation of dust or prolonged skin contact poses low but real irritation risks. Industry training points out storage requirements: sealed containers, stable temperatures, and avoidance of strong acid or oxidizers. European REACH regulations and OSHA workplace standards outline clear disposal steps. Recent attention to worker welfare and green chemistry motivates investments in exposure monitoring, spill containment, and emergency planning.
Buffering pharmaceutical formulations stands at the top of the list for AMP use, regulating salt content and pH for stable drug performance. Cosmetic chemists prize it for balancing acidity in creams and lotions, lending smooth textures that don’t sting the skin. In the industrial field, waterborne coatings make use of the compound’s solubility and pH control to enhance paint consistency without harsh volatile ingredients. Fiber and textile manufacturers integrate it during dyeing processes, gaining even coloration. Biotech companies incorporate the substance in DNA extraction protocols and cell culture systems, taking advantage of its gentle pH modulation. Electronic industries include AMP during solder mask fabrication, protecting sensitive circuits in printed circuit board production.
Academic groups push the boundaries further, probing structure-activity relationships with AMP derivatives to discover new antimicrobial or anti-inflammatory effects. Some teams explore its role as a chiral auxiliary, aiming to improve steric control during synthesis of optically active compounds. Investigations into green synthesis look for renewable starting materials and energy-efficient catalytic routes, which could shift reliance away from hazardous legacy processes. Startups look into AMP-modified polymers for biodegradable packaging, and studies benchmark water retention and physical properties for use in 3D printing.
Toxicological studies clarify comfort zones for AMP use in consumer products. Acute toxicity registers low for oral, inhalation, and dermal exposure in animal studies. The compound does not easily cross biological membranes, and rapid excretion leads to minimal bioaccumulation. Some irritation emerges with concentrated solutions, warranting controlled exposure in cleaning, medical, or laboratory applications. Epidemiological monitoring has yet to flag serious long-term risks, though scientists track metabolites for any connections to chronic toxicity, especially as regulatory frameworks push for ever-safer chemical choices in household and personal care items.
Companies exploring next-generation manufacturing keep their focus on compounds like AMP, since simple amino alcohols can replace harsher chemicals in processes demanding both reactivity and safety. Biomaterials research circles back to such building blocks due to their reliability in stabilizing proteins or enhancing solubility of new therapeutics. Innovation efforts gear toward higher-purity production using biocatalysis, which could cut waste and energy consumption compared to petrochemical-based paths. The search for more sustainable industrial chemistry will likely boost AMP’s footprint, with novel derivatives entering new areas of medicine, electronics, and consumer science.
2-Amino-2-Methyl-1,3-Propanediol—widely known as AMP or AMP-98—doesn't often make headlines, but its reach stretches into plenty of everyday products. In labs, this ingredient turns up as a buffer. Scientists use buffers to help keep the pH of a solution stable, which matters in everything from medical research to industrial processing. An unstable pH can throw off chemical reactions, mess with data, and ruin batches of products. I remember a time during a college chemistry experiment where forgetting the right buffer led to hours of wasted work and some interesting colors that didn’t tell us anything useful. That experience sticks with me, a reminder of how one overlooked component can mess up a bigger system.
Outside the lab, AMP finds its place in cosmetics and skin care. Many moisturizers and cleansers use AMP to balance their formulas. In cosmetics, pH means a lot. Too high or too low and you could face irritation, breakouts, or simply a product that doesn’t do what it promises. AMP keeps things steady. According to research published in the journal Cosmetics, ingredients like AMP allow better texture, shelf life, and user safety, proving its value without flashy advertising. Having sensitive skin and trying out different creams over the years, I grew wary of products causing redness. Formulas balanced by ingredients such as AMP seem to cut down on those unwelcome reactions, at least for me and plenty of folks I've spoken to.
Another place AMP shows up: the coatings industry. Manufacturers count on AMP to improve paint performance and stability. When you paint a wall, ingredients like AMP help reduce lumps and streaks. You get a smooth layer that lasts longer. Research by the American Coatings Association points out that buffering agents can also help reduce the need for harsher chemicals. That small substitution matters for workers’ safety and indoor air quality at home. Having spent a summer painting apartments, I saw how the right paint formula saved tons of time and hassle—no one likes redoing a wall over sticky spots or faded patches a month later.
AMP also finds a foothold in water treatment. Purifying water isn’t just about catching big particles. Chemical stability helps prevent dangerous byproducts from forming. Cities and industries rely on this, especially given regulations from groups like the EPA—the Environmental Protection Agency. If water pH wobbles outside safe limits, pipes corrode faster and certain contaminants become tougher to remove. Using AMP as a buffer contributes to lower maintenance costs and safer drinking water. This might sound far removed, but for anyone who's dealt with rusty tap water or boil advisories, the value becomes immediate.
With all these uses, one concern rises to the top: environmental impact. Any widespread chemical, especially one tied to water treatment and industrial paints, draws attention from researchers and regulators. Questions about biodegradability, toxicity for aquatic life, or micro-pollutants come up. Regular reviews by health and environmental bodies check for new risks, and manufacturers have to keep up with safer practices or tighter restrictions. It’s best to watch for independent safety data and updates, not just rely on marketing claims. For people working with AMP or exposed to it indirectly, basic handling rules and keeping an eye on ongoing research forms part of a responsible approach.
AMP gives a glimpse of the often invisible world of specialty chemicals. We see finished products—a favorite lotion, clean tap water, a well-painted wall—but the ingredients holding them together matter at every step. Careful review, transparent data, and open conversation between scientists, industry, and the public offer the best route to keep products safe, workers protected, and our environment respected.
I’ve spent time in both academic and production labs, and every chemical brings its own challenges. 2-Amino-2-Methyl-1,3-Propanediol (sometimes called AMP or AMP-95) looks simple enough. It shows up in biochemical buffers and a range of manufacturing processes. The catch lies in handling. The transparency and almost benign appearance can lull people into dropping their guard, and that’s where mistakes begin.
Pulling on gloves isn’t just a habit — it’s a non-negotiable step. The stuff can irritate skin, dry it out, and cause redness. Gloves that offer chemical resistance, such as nitrile, keep you safe from unexpected splashes. My colleagues and I treat goggles as required equipment. Eyes take a serious hit from this compound, as it has a way of causing irritation quickly if a droplet finds its mark. Even seasoned techs skip the risk by grabbing face shields for messy dilutions.
I remember someone prepping a batch without considering airflow, which led to eye and throat discomfort. The odor isn’t always strong, so you can expose yourself before realizing what’s happening. Good chemical work involves local exhaust or fume hoods. Ventilation takes on real meaning when you’ve heard stories of people developing headaches and coughs in poorly ventilated storerooms.
Handling starts with storage. Keep AMP in a dry, well-labeled container with tight fits to prevent moisture from creeping in. Moisture alters concentration and can trigger decomposition over time. I once opened a loosely capped bottle to find the contents had crusted and changed color—not good. Consistent labeling with clear hazard symbols stops mix-ups, especially during busy shifts.
Accidents teach lessons, sometimes the hard way. If AMP spills, speed matters. Reach for absorbent materials and non-sparking tools, scoop carefully, and bag waste for designated chemical disposal. Flushing with water, especially after skin contact, helps avoid burns or severe irritation. Eyewash stations and emergency showers need to be operational and accessible, not blocked by boxes or equipment.
Experience only means so much. Fresh workers and seasoned staff alike must review safety protocols regularly because procedures sometimes shift with new research. I’ve seen labs drill basics every few months so nobody gets complacent. Regular reviews help clear up confusion, especially when storage temperatures or compatibility charts change.
A strong lab safety culture relies on people calling out unsafe practices without fear of being ignored. If someone skips goggles, a quick reminder often does more than any poster on the wall. It’s not about catching mistakes—it’s about building habits everyone can trust. Automated inventory and digital chemical logs support those habits by keeping expiration dates and usage history visible.
Research points to safer alternatives for pH adjustment and buffering, and some operations choose automated systems to minimize direct handling. Automation takes people out of the spill zone and reduces exposure risk. Manufacturers who share updated safety data sheets and support ongoing lab education help users stay ahead of emerging risks.
2-Amino-2-Methyl-1,3-Propanediol. Sounds complex on the surface, but its chemical formula, C4H11NO2, breaks it down to the basics: four carbon, eleven hydrogen, one nitrogen, and two oxygen atoms. That's the main structure chemists point to, whether working at the lab bench or updating an MSDS sheet. Don’t let the string of syllables intimidate you. Plenty of folks in science gave this compound a nickname—AMPD.
I run into a lot of folks who think formulas only matter to those wearing lab coats. Truth is, those scientific shorthand codes appear in everyday products and water treatment systems. Knowing C4H11NO2 showed up once during a routine water pH adjustment at our local plant, I realized just how much these chemicals draw invisible lines between research, safety, and clean resources.
You can spot AMPD in buffer solutions—often as a pH stabilizer in processes that keep pharmaceuticals or research samples steady. If that pH shifts, so can entire batches of medicine or research findings. This isn’t just trivia for chemistry whizzes. This is about the reliability of what ends up in your medicine cabinet or municipal drinking water.
Experience tells me trust builds when information gets shared straight. The formula C4H11NO2 isn’t just numbers and letters; it’s shorthand chemists and manufacturers rely on for managing risks. If an emergency strikes—say, chemical exposure at a plant—first responders need exact details to treat an incident. Mixing up formulas has, historically, led to costly mistakes and health risks. The safety data gets clearer and more accessible when people recognize the shapes of molecules they're working with.
Looking for AMPD, you’ll run into grading differences. Lab supply companies sell both "reagent" and "industrial" grades. Purity varies, and impurities can spell trouble—especially in pharmaceutical or food-use scenarios. Once, we tracked down a contamination issue at my previous workplace. We found it came from an unlabeled drum, later tested as a lower-grade version of AMPD. The slip-up stalled production and left several teams scrambling to fix procedures for ordering and storage.
Supply chains tighten up when chemical identity and purity stay front and center. This doesn’t just protect workers. Regulators have a field day when tracking consistent chemical labeling—especially after a few high-profile contamination scares a decade back. Industry and authorities both do better with transparency and clearer documentation at every step.
Simple awareness of what goes into everyday chemical formulas might not grab headlines, but it pays off in safety and reliability. I’ve seen how clear labeling and better recordkeeping mean fewer workplace injuries, lower insurance costs, and steadier product quality. Reading about C4H11NO2 shows the importance of easy access to trustworthy information, not only for experts but for everyone who relies—sometimes unknowingly—on its benefits.
Walking into most labs, strange names pop up across bottles and drums, but every chemist knows there’s a rhyme and reason to the chaos. 2-Amino-2-Methyl-1,3-Propanediol, or AMPD, always pops up on that list of compounds you do not just toss on the nearest shelf. In my own experience, even in bustling, overstocked labs, routine care stops accidents before they start.
Physically, AMPD takes form as a white or almost white crystalline powder. It looks harmless, but even familiar chemicals can turn risky with bad habits. One wrong move—store it in the sun, leave a lid loose, let moisture sneak in—and you turn convenience into an incident report. Science never coddles complacency.
AMPD pulls in water faster than most newcomers expect. It acts as a desiccant, a thirsty magnet for humidity. Open that jar during a sticky summer, see how quickly it clumps together or liquefies. Moisture takes a toll not just on appearance, but purity and reactivity as well. Spoiled or contaminated reactants mean ruined experiments, wasted hours, and at worst, dangerous results.
So, dry storage means more than a preference—it’s non-negotiable. I’ve always looked for airtight, well-sealed containers—glass over thin plastic if possible. Desiccators (those trusted dome jars with drying agents inside) keep the contents bone dry until you need them. In student labs, too many times I’ve seen AMPD left out just for a minute on a humid day, then back in the bottle without a thought. Later, those same students wonder why their syntheses fall apart or their pH drifts off target.
Keep AMPD tucked away at room temperature, but here’s the trick: go for the lower end. Not freezing or close to zero, just never in the path of heaters, sunlight, or vent blowers. Temperature swings tweak stability, so the back of a bench away from machines works best for me. Warehouses need more structure, so controlled stores or clearly marked cabinets give peace of mind. Heat can drive decomposition, chemical changes, and unexpected pressure in containers.
Labels fade, bottles move, but habits matter more. Make sure everything gets closed tight right after use. No excuses. Many incidents from my grad school days came from an “I’ll put it back later.” Those tiny oversights grow into big problems. I once lost a week of work because our AMPD bottle sat open for just one afternoon, the humidity did the rest.
Each time you transfer AMPD, scoop out powder or weigh it, clean tools stop contamination. Mixing chemicals—intentionally or by dirty spatulas—can lead to wild, unpredictable reactions. Never share scooping tools across bottles. It sounds simple, but shortcuts drive the worst mishaps.
OSHA and chemical suppliers always spell out best practices, but top-down rules can’t beat a lab team’s own routine vigilance. Quick habit checks, spot training for new lab staff, and setting the right example pay huge dividends. One sharp technician with eyes on the basics keeps the whole operation safer and much more productive.
2-Amino-2-Methyl-1,3-Propanediol deserves a careful corner of the lab, where dry air and cool temperatures keep it reliable. Safe storage does not take fancy equipment or elaborate protocol, just daily discipline and a healthy respect for even the most familiar substances.
People working in labs or factories might know 2-Amino-2-Methyl-1,3-Propanediol by its other name, AMP-95. It shows up in a stack of products — everything from metalworking fluids to cosmetics and even some coatings. The stuff helps adjust pH and lets different chemicals play nice together. It’s colorless and doesn’t smell, so it slips through life pretty easy. But those who work with chemicals always end up asking the same thing: is it safe for people and the planet?
Get this on your skin and, for a lot of folks, it causes redness or a bit of a rash. Eyes seem to take the brunt, burning and watering if a splash lands the wrong way. Breathing in the dust or vapor isn’t common, but it can irritate your nose and throat. I’ve spoken with plant workers who describe prickle or itching after long days handling drums. The science points to AMP-95 being an irritant, not something that sets off bigger alarm bells like cancer or nerve damage.
Swallowing brings its own risks. Even small amounts can upset the stomach, causing pain or nausea. Specialists who work on chemical safety recommend gloves and goggles, which seems like common sense, but corners sometimes get cut on busy lines or in tight labs. I’ve seen strong safety cultures make a big difference; training and good habits seem to keep most accidents at bay.
Many folks don’t think past the bottle or barrel, but runoff and disposal shouldn’t get ignored. 2-Amino-2-Methyl-1,3-Propanediol breaks down pretty well in water and soil. Microbes munch it up, so it doesn’t often pile up in streams or lakes. Even so, spills at factories or storerooms can raise the pH of water, and fish just aren't built for sudden changes like that. High doses can hurt aquatic critters, though the risk from normal use—think, diluted down the drain—looks small when you stack it against petroleum spills or heavy metals.
Having grown up near creeks and seeing how quickly one chemical drum turns clear water into a fish kill, I believe everyone needs to think about safe handling and smart disposal. Many countries set limits for wastewater discharge, but enforcement still depends on who’s watching and how much cash is on the line. Blow it off, and you end up with sick rivers.
Most issues link back to direct exposure or poor housekeeping. Workers need protective gear, good ventilation, and clear procedures for spills. Factories need waste systems that catch runoff and treat it, rather than pumping it into the ditch. These things cost money, but fines and long-term cleanup cost more.
Some firms look for alternative chemicals that do the same job with fewer downsides. I’ve seen green chemistry projects test amino acids or other natural bases, though swapping out a tried-and-true ingredient takes years and loads of patience. Until greener options stack up, everyone using 2-Amino-2-Methyl-1,3-Propanediol just has to respect it, follow the rules, and keep safety gear handy.
After talking with both safety engineers and people on the line, it’s clear: training changes outcomes. Good programs mean people know the risks, how to avoid them, and what to do if something goes sideways. Regular drills, honest reporting, and attention paid to small leaks or spills keep bigger problems out of the news. The bottom line — it takes effort, attention, and a bit of humility to keep chemicals useful without letting them cause trouble.