Back in the mid-20th century, scientists in labs across Europe and the United States scrambled to find stable and dependable chemical buffers for biochemical research. It was during this period that 2-Amino-2-hydroxymethyl-1,3-propanediol, widely referred to as Tris or THAM, found its calling. ESR and NMR studies in the 1960s demonstrated Tris’ impressive ability to maintain consistent pH levels without interfering with sensitive molecular structures. The story behind Tris highlights the classic drive for practical solutions in molecular biology, where laboratory experience shows that sometimes a simple, robust molecule can open up entire lines of inquiry.
In daily research, Tris has become nearly synonymous with every molecular biologist’s basic toolkit, much like a pipette or buffer solution. It’s colorless, crystalline, and dissolves quickly, lending itself to tasks from DNA extraction to protein isolation. Its pKa, around 8.1 at 25°C, fits like a glove for biological systems that need to hover near physiological pH. Tris never gets the spotlight, but anyone who’s ever run an electrophoresis gel or diluted cell culture media owes some respect to this underappreciated backbone of modern biochemistry.
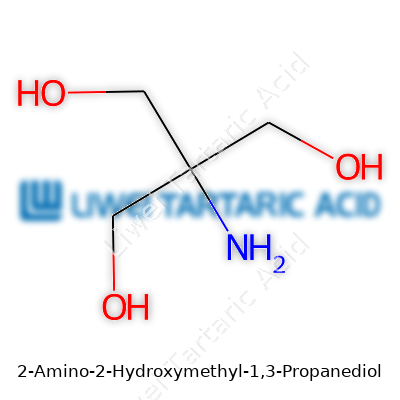
Tris stands out for its unique set of features in a world filled with reagents begging for attention. It’s solid at room temperature, crystalline, with a characteristic mild sweetness if you catch its dust on your tongue—something old-school chemists will remember, though modern safety protocols frown on such casual sampling. The molecule's structure includes three alcohol groups and a primary amine, making it highly soluble in water and capable of neutralizing acids efficiently. Its melting point hovers near 167°C, ensuring stability in the face of routine heating or sterilization procedures. The chemical formula—C4H11NO3—makes clear why it’s sometimes called tromethamine: it packs nitrogen and oxygen with a balance that enables broad chemical compatibility.
Anyone scanning the side of a reagent bottle in a well-stocked lab will find Tris labeled with purity ranging from molecular biology grade to USP for clinical use. Labels typically give a CAS number (77-86-1), molecular weight (121.14 g/mol), and storage advice to keep it cool and dry. The Material Safety Data Sheet (MSDS) usually highlights low acute toxicity but points out that prolonged inhalation or skin contact isn’t a good idea. Professionals handling Tris in bulk—maybe in pharmaceutical manufacturing or dialysis—see technical sheets filled with heavy detail on particle size, moisture content, and assay specifications so the buffer doesn't sabotage downstream applications.
Preparing Tris buffer starts in the fume hood, measuring crystalline Tris base into distilled water, gently swirling until everything dissolves. Most protocols come down to tweaking the pH with concentrated hydrochloric acid or sodium hydroxide while monitoring with a calibrated pH meter. Longtime lab workers know to dial in the pH at working temperature because temperature shifts can throw the pH out of range. Many labs filter-sterilize their solutions using 0.22-micron filters to avoid clogging microbial growth later on. Industrial producers use controlled environments to synthesize Tris via the condensation of ammonia with nitromethane, followed by reduction and purification, yielding kilogram quantities of consistently pure product.
Tris isn’t just a bystander in solutions; the functional groups come alive in the presence of reactive chemicals. Its hydroxyl groups can get esterified or oxidized if you’re creative and need to attach labels or modify reactivity. The primary amine can participate in acylation reactions, amide bond formation, or even conjugation to biotin or fluorescent tags. Veterans in protein chemistry caution that Tris can react with compounds like formaldehyde, so choosing the right buffer depends on everything else happening in your tube. Microbiologists have learned to avoid using Tris buffers with certain DNA-modifying enzymes, since some will show reduced activity or altered specificity.
On shelves and catalogs, Tris goes by a variety of monikers. Some sell it as tromethamine, trometamol, or Trizma, depending on the supplier or local regulatory code. In the clinic, doctors prescribe THAM as an injectable for acid-base imbalances. Researchers in more niche fields may stumble across derivatives labeled as tris(hydroxymethyl)aminomethane or refer to it by formula names. With so many variants, seasoned chemists double-check product codes and chemical structures before ordering bulk quantities, especially for clinical or GMP-compliant manufacturing.
Research teams that take safety seriously follow procedures to the letter. Even though Tris ranks low on acute toxicity, powder spills raise dust, which can irritate lungs or eyes. Standard lab practice means gloves and goggles, and workers know not to eat or drink near the bench. I’ve seen environmental health and safety officials harp on proper waste disposal, since buffer rinses can slip unnoticed into the sink. In pharmaceutical and clinical settings, adherence to cGMP production, batch traceability, and purity assurance protects both patients and supply chains. Because Tris-based solutions often go into diagnostics or even direct patient care, the stakes demand this vigilance.
Every scientist in a biomedical lab brushes up against Tris: It balances pH in buffers for DNA, RNA, and protein extraction. Serves as the backbone of electrophoresis running buffers. Steps up as a component in cell and tissue culture media. Clinical teams rely on its injectable form in emergency medicine to counteract severe acidosis, especially when other treatments fail or take too long. Industrial biomanufacturers count on Tris to stabilize biologics during purification or to keep enzymes humming during production. Cosmetic chemists formulate Tris-based creams that need reliable pH balance for sensitive skin. Its flexibility makes it the quiet engine behind so many research accomplishments in genomics, proteomics, and clinical investigation.
Improvements in Tris production and application rarely make headlines, but they shape experimental reproducibility worldwide. Groups are constantly testing tweaks to buffer formulation that might enhance enzyme stability or reduce unwanted chemical side reactions. Techniques like real-time pH mapping allow scientists to tailor Tris-based buffers to the needs of single-cell analysis or high-throughput screening. Pharma R&D teams evaluate whether higher purity grades or new derivatives could boost shelf life in injectable pharmaceuticals. Bioinformatics tools now assess historic batch inconsistencies and recommend protocol adjustments so researchers avoid repeating old mistakes. These advances translate into smoother workflows and greater confidence in published results.
Public health experts and toxicologists don’t let the relative safety of Tris lull them into complacency. Studies in cell lines and animal models have tracked metabolic fate and potential for acute or chronic effects. Routine use hasn’t flagged any significant carcinogenic or mutagenic risks. The compound’s main hazards involve potential to depress respiratory or circulatory function at obscenely high doses, which basically never come up outside clinical emergency use. Nevertheless, continuous review remains critical once Tris enters clinical pipelines, especially as an excipient in new biologic drugs. Environmental scientists monitor wastewater for buffer remnants — evidence that widespread use, even of low-toxicity chemicals, accumulates consequences over decades.
Looking toward the future, Tris isn’t likely to fade from the scene. Demand will rise as personalized medicine and global biotech infrastructure expand. Research into greener synthesis routes could shrink the environmental footprint, a concern as labs worldwide struggle with sustainability mandates. Analytical chemists talk about “smart buffers” — new generations of Tris derivatives that respond dynamically to temperature or ion strength. Integration with digital lab management systems could automate buffer preparation, freeing up hours for actual discovery work. Ongoing vigilance in quality control and safety will ensure Tris lives up to its proven track record, helping scientists and clinicians push boundaries safely and effectively.
In most scientific labs, 2-Amino-2-Hydroxymethyl-1,3-Propanediol usually goes by the much simpler name "Tris" or "Tris buffer." For researchers and lab techs, this stuff feels as familiar as coffee. Anyone who’s spent time testing water samples, tweaking DNA extractions, or prepping cell cultures has likely handled Tris. With its solid ability to keep acidity in check, it’s a staple for solutions like electrophoresis buffers and cell incubations. Getting pH just right means experiments don’t end up ruined, and results stay reliable. Mess up the pH, and weeks of work can end up in the trash.
One of the main reasons everyone reaches for Tris is its knack for handling biological samples without messing with the stuff inside. It keeps proteins happy, maintains the structure of DNA, and doesn’t react much with other things in the mix. In my own experience running protein gels or PCRs for student projects, a fresh Tris buffer always meant the experiment had a solid shot at working out. Skimping on it or grabbing a cheap knockoff usually led to fuzzy results and a scramble to troubleshoot.
I’ve also seen how labs use Tris to keep things steady for bacteria and yeast. The buffer lets cells do their work while researchers track metabolism, enzyme production, or monitor environmental toxins. Some wastewater treatment plants even tap into simple chemistries like Tris for field-testing pollution, because it helps stabilize everything long enough for readings to matter.
Over 100,000 research papers mention this compound by name, and behind those stats sit thousands of grad students and scientists relying on that familiar buffer. In water treatment, Tris helps prepare samples for accurate pH and ammonia testing, which affects how cities set safety limits and keep drinking water healthy. Hospitals and clinics depend on Tris in labs that diagnose infections, from throat swabs to blood samples.
Manufacturers use Tris as well, not just in test kits. Cosmetic brands add it to creams and shampoos to keep the skin’s feel balanced and formulas stable. Some companies that make sunscreen or lotions pick Tris so their products don’t sting or throw off skin tone.
Tris delivers a lot, but it comes with its share of headaches. At higher temperatures, the buffer doesn’t hold pH steady. I’ve dealt with this personally during summer months, watching experiment results drift because the lab’s AC couldn’t keep up. This weakness pushes scientists to either add temperature controls or switch to pricier alternatives, both of which eat into budgets.
Some research points to the need for greener, safer buffers. Disposing of old Tris or spillages poses minor hazards, and a few groups are searching for eco-friendly versions. Green chemistry pushes everyone to rethink waste, and tighter controls in labs could help reduce environmental impact.
Teams that use Tris every day can make the most of it by using clear training and regular checks to avoid common mistakes. Rotating stock, tightening waste policies, and storing Tris away from the heat pays off in lab performance. Companies keep investing in better shipping and packaging to cut spills. Some startups are experimenting with buffer systems that break down more quickly in the wild, hoping to reduce risks while keeping performance high. Staying informed about new research and updated safety protocols can help everyone use 2-Amino-2-Hydroxymethyl-1,3-Propanediol with more confidence and less impact.
Every lab I’ve stepped into has one thing in common: chemical containers lined up like chess pieces, each with a label, a story, and a risk. 2-Amino-2-hydroxymethyl-1,3-propanediol, better known as Tris or Tris buffer, usually sits among them. Most researchers come across this compound by the kilo, since it buffers many solutions in biology and chemistry labs. But just because it’s common, that doesn’t mean everyone knows the right way to handle it.
At first glance, Tris doesn’t scare anyone. It looks like any other white crystalline powder. Safety data sheets (SDS) flag it for mild irritation. Breathing in dust, splashing it on bare skin, or dropping it in your eyes can make you uncomfortable—burning, stinging, or even sneezing for hours. Each pinch that lands on my glove reminds me not to touch my face or wipe sweat from my brow.
Tris won’t explode, but it can throw off your day. Long-term inhalation brings up questions about chronic respiratory irritation, though direct links to long-term illness are thin. There’s no known cancer link, and it doesn’t build up in your body, but that doesn’t mean risks vanish. If the powder gets airborne, you can taste it—your mouth tingles, and your nose itches. It’s a jolt to realize you forgot the dust mask for a routine buffer prep.
Legitimate chemistry comes with real-world risks, even for “safe” substances. Tris regulations are less strict compared to corrosives or toxins, but safe handling isn’t just about following rules. A high school teacher once said, “Familiarity breeds carelessness.” I’ve seen too many smart people forgo gloves since “it’s just Tris.” Minutes later, they’re lined up at the sink rinsing their hands. Accidents don’t pick favorites; one lapse in judgment can bring consequences.
The Centers for Disease Control and Prevention warn that irritation from direct contact isn’t rare. The American Conference of Governmental Industrial Hygienists doesn’t even give it a strict exposure limit, but lab managers still demand gloves, eye protection, and clean benches. I learned from a missed eye shield—the burning lasted an entire afternoon. That lesson sticks more than any textbook example.
To cut down on risk, splash-proof goggles, a good pair of gloves, and dust masks play a big role. The powder may feel routine after years in the lab, but all it takes is an open container and a stray elbow for a cloud of dust to make its way to your face. Hood work or using a fume extraction arm when weighing helps, especially in cramped spaces.
The Environmental Protection Agency doesn’t list Tris as a huge environmental threat, but rinsing buffer waste straight down the drain without dilution piles up unnecessary hazards. Careful labeling, storage on lower shelves, and not working alone with chemicals protect both the seasoned technician and the new starter. Common sense—rarely prescribed, often ignored—should guide every chemical transfer.
Trust in these established safety habits keeps any workplace honest. Tris isn’t a monster, but it doesn’t need to become one either. Respect for the risks, even when it feels like busywork, keeps the worst days from happening. Cafeteria conversations about buffer splashes make an impression, long after the irritation fades. Every lab story is a reminder: what you do each day shapes tomorrow’s safety record.
TRIS, or 2-Amino-2-Hydroxymethyl-1,3-Propanediol, pops up in so many biochemistry labs that it almost fades into the background. Scientists routinely reach for its familiar bottle when setting up experiments that depend on stable pH conditions. Calculating the precise amount depends on knowing its molecular weight: 121.14 g/mol. That number isn’t just trivia. It ripples through every step of a buffer preparation, quietly dictating whether experiments will run smoothly or barely get off the ground.
Precise measurements make all the difference in both routine and groundbreaking experiments. Picture someone preparing a liter of buffer for a protein purification run. Even a small miscalculation skews the concentration, pushing the pH out of the sweet spot and triggering unpredictable results. Reagents like TRIS set the foundation for sensitive studies—from DNA extraction to enzyme kinetics. Researchers trust that 121.14 grams added to a liter of solution creates exactly one molar concentration. That’s not just chemistry; that’s hard-earned confidence built on reliable numbers.
TRIS stands out thanks to its chemical makeup. Its formula—C4H11NO3—packs a lot of punch in a compact molecule: four carbon atoms, eleven hydrogens, a nitrogen, and three oxygens. Each atom steps onto the scale: carbon at 12.01, hydrogen at 1.01, nitrogen at 14.01, and oxygen at 16.00. Running the math, the sum gives 121.14 g/mol, confirming the value chemists rely on. Reliable suppliers and quality benchmarks keep this value tightly consistent, so every vial on a shelf lines up with protocols penned decades ago.
While TRIS dominates the world of molecular biology and biochemistry, its uses don’t end there. It supports diagnostic laboratories, water treatment facilities, even certain pharmaceutical formulations. Buffering power and relative safety put it in the good graces of regulatory bodies. That molecular weight follows TRIS into every application, still guiding the hands of technicians mixing doses or scaling up reactions for industry applications.
Every field faces its speed bumps, and chemistry is no exception. Not every reagent comes pre-packaged with perfect instructions, and lab staff might rush in busy environments. Digital balances and online calculators help, but training counts just as much. Getting people comfortable with calculating molar concentrations, double-checking values, and recognizing outdated or impure stock keeps labs productive and safe.
Open communication across teams shrinks avoidable mistakes. Techs catch a mistyped value before it triggers a cascade of ruined samples. Students spend time cross-referencing trusted sources or peer-reviewed literature for TRIS’ specs rather than falling for quick internet search errors. Even dried-out bottles prompt a community check on quality, not just a rushed refill.
Investing in regular training improves accuracy. Lab managers who update protocols and offer quick-reference guides for reagents like TRIS see fewer mistakes and lower waste. Pushing for better digital recordkeeping helps, too. When staff document preparations and double-check molecular weights, no one has to wonder about the background of a buffer used weeks before.
Institutions can source TRIS from reputable suppliers whose documentation matches international standards. Regular lab audits catch outdated bottles and keep everyone in sync. Relying on numbers like 121.14 g/mol, not just because tradition demands it but because accurate chemical handling builds trust, shapes careers, and keeps research moving forward one solution at a time.
2-Amino-2-Hydroxymethyl-1,3-Propanediol, also known as Tris or Trometamol, gets used all the time in biochemistry labs, pharmaceuticals, and even cosmetics. Safe storage rarely makes headlines, but ignoring storage basics can invite trouble in labs and on production floors. Anyone who’s worked with chemical buffers or handled reagent prep knows how easily spills and spoilage happen without good habits.
Tris usually comes as a white crystalline powder. The first thing anyone notices is how it likes to soak up moisture from the air. Keep it exposed for long, and it’ll start clumping or caking. That spells frustration for researchers needing reliable, easy-to-weigh portions. More than that, humidity and direct sunlight slowly chip away at the compound’s quality over time. Letting moisture creep in might not seem dramatic, but it opens the door to slow degradation, unpredictable reactions, or changes in pH during sensitive experiments.
It always helps to keep Tris in tightly sealed containers. Screw-cap bottles with gasket seals work better than basic snap lids. From experience, glass works fine if there’s no risk of breakage, but high-quality plastic bottles resist accidental drops a bit better. If someone stores the powder in the original packaging, it’s smart to inspect the lid every time after use. Forgetting this step has tripped up plenty — one loose cap can turn fine, free-flowing powder into unusable lumps.
Humidity cuts shelf life. Most suppliers recommend dry, cool, well-ventilated shelves. In humid zones, a simple desiccant packet tossed into the storage bin works wonders. Avoid keeping any lab chemicals near heat sources, windows, or anywhere sunlight blasts the shelf. Sunlight can make plastic containers brittle or speed up chemical decay.
Tris does find its way into products beyond research. Sometimes it’s in creams, gels, or stabilizers in commercial goods. While pharmaceutical manufacturing handles bulk volumes, end-users at home won’t likely face the same risks. Still, even household quantities stay safest inside their original container, snapped shut and away from curious hands.
Anyone who’s spent hours running gels or preparing solutions learns the value of careful storage. More than once, I’ve reached for an old chemical jar only to find a crusty mess. In some labs, people label powders with the date opened, which helps flag containers that lived past their best-before period. Rotating stock keeps accidents rare — it’s tempting to reach for an old bottle at the back, but fresher material delivers more predictable results.
Thinking ahead keeps labs running smoothly and protects budgets over the long haul. Poorly stored chemicals lead to more waste and make labs less reliable for everyone. Simple habits — sealing jars, using desiccants, noting open dates, storing in cool dry places — beat high-tech solutions for most small facilities. For larger production sites, environmental monitoring and climate control make even more sense.
Safe storage of Tris isn’t just following rules; it’s part of working smarter and safer. Fewer surprises, cleaner experiments, and lower risk of accidental waste benefit everyone, from the college research student to the pharmaceutical pro. A little day-to-day care up front cuts bigger headaches down the road.
In most labs, few chemicals pull as much weight as 2-amino-2-hydroxymethyl-1,3-propanediol. Folks call it TRIS, and it’s a backbone for everything from buffer solutions in biochemistry to fundamental experiments in molecular biology. If you have ever handled a protein, DNA extraction, or even everyday pH balancing, you’ve dealt with this stuff. This compound solves one key problem—keeping pH steady even if the experiment tries going off the rails.
One thing always comes up before anyone thinks about mixing a solution for an experiment: Will this stuff dissolve in water? For TRIS, the solubility happens to be pretty forgiving—about 500 grams per liter at room temperature, according to Merck and Thermo Fisher reference sheets. This value lands TRIS among the most water-soluble substances sitting on the biochemist’s shelf. For perspective, putting that much salt or sugar in a liter of water leads to a sludgy mess, but TRIS just disappears in and creates a clear solution. You do not see undissolved powder, no matter how much you add, up to that limit.
High solubility means less hassle in the lab. Weigh the powder, pour, stir, done. Most labs run experiments needing variable buffer concentrations, from a few millimoles per liter for sensitive work up toward half a mole per liter for demanding protocols. TRIS’s ability to dissolve in practically any amount used gives researchers flexibility. No special equipment, no warming, just pure convenience. This seems small until you’re working late or under stress—nobody enjoys fighting with floating clumps or heating stubborn powders when there are already a dozen other things to juggle.
For busy lab technicians or grad students, this kind of straightforward solubility means more reliable results. Poorly dissolved buffer powders will have hotspots—sections of solution where concentrations go all over the map. That introduces guesswork and errors, especially for work that depends on exact pH ranges. Consistent dissolving knocks out one whole category of troubleshooting, so experiments get done on time, and reproducibility stays high. Researchers chasing experiments for days know the pain of unstable buffers kicking projects back to square one.
It always pays to think about risks. Some chemicals, even if they dissolve well, produce nasty byproducts or show toxicity in waste streams. TRIS stands out here too. Most safety data points to low toxicity and ease of disposal. Used buffer solutions usually run right down the drain in most countries, without requiring hazardous waste containers. This trait puts less pressure on fee-strapped university labs and biotech startups working on a budget.
Wastewater management teams breathe a little easier, too. TRIS in environmental studies dissolves quickly, doesn’t stick around, and does not accumulate in plants or animals the way heavy metals or organic toxins do. As regulations tighten and climate risks rise, chemicals that avoid long-term groundwater contamination or eco-toxicity win out.
If any problems show up—maybe precipitation forms if the water contains too many impurities or the temperature drops—simple steps clear things up. Filtration through a 0.2-micron filter or gentle heating makes sure every last bit gets into solution, saving effort and improving results. For large-scale applications, lab managers switch to pre-made liquid TRIS or bulk-powder setups with automated mixing tanks, reducing manual error and waste.
Solubility sets the stage for everything else in the lab. As researchers ask more from their experiments, relying on proven basics like TRIS and trusting in its straightforward behavior saves time, money, and worry. Whether prepping a buffer for PCR, stabilizing a sensitive protein, or running day-to-day tests, knowing a chemical just works—fast and without fuss—makes all the difference.