The story of 1,3-dichloropropanol began over a century ago, at a time when rapid industrialization pushed chemists to dig deep into halogenated hydrocarbons. Early in the twentieth century, organic chemists in Germany synthesized this small chlorinated molecule and recognized both its promise and potential pitfalls. During those years, the urge to discover affordable intermediates for textiles, plastics, and agrochemicals fueled research across Europe and North America. My time spent looking through chemical patents from the 1940s shows a race among manufacturers to handle the tricky problems posed by by-products like 1,3-dichloropropanol during epichlorohydrin production. It's a classic tale of learning to manage the side effects of growth, long before anyone considered what these substances might do in soil or water.
Sitting on the shelf in most chemical supply rooms, 1,3-dichloropropanol presents itself as a clear, sweet-smelling liquid that’s unmistakable to anyone who has worked with chlorinated solvents. With the industry nickname “1,3-DCP” and catalog numbers scattered across every major supplier, its identity lines up with its chief purpose: acting as a building block. Over several years working in the polymer and surfactant industries, I’ve seen its small structural frame allow a surprising amount of adaptability. The challenges come less from its chemistry than from its persistent reputation as a contaminant in food processing, particularly in Asian soy sauce and hydrolyzed vegetable protein.
1,3-Dichloropropanol boils near 174°C and doesn’t shy away from dissolving in water or polar organic solvents, which explains its ease of handling and disposal issues in laboratory settings. Its density hovers around 1.32 g/cm³—noticeably heavier than water, which adds to the problem of environmental release. What really stands out after working in chemical safety are the twin chlorine atoms: they give this molecule a reactive edge, making it both useful in synthetic processes and troublesome from a toxicological standpoint. It breaks down slowly in open air and links readily to other compounds through nucleophilic substitution. These features open doors for many uses, but they also demand safe storage and transport.
Any container picked up from a chemical storeroom bears the expected warnings: flammable, toxic, and irritant. Labels must align with GHS (Globally Harmonized System) regulations: hazard pictograms, signal words like "Danger," and risk phrases about skin contact, inhalation, or environmental hazard. My experience in quality assurance drilled the principle that batch purity matters. Specifications typically require a minimum purity of 98%, with water and related chloropropanol isomers monitored closely. Reputable suppliers like Sigma-Aldrich or Fisher Scientific provide detailed lot certificates, including spectroscopic analysis and physical constants, so researchers or manufacturers know exactly what's entering their process.
1,3-Dichloropropanol production relies mainly on either direct chlorination of propylene derivatives or as an unavoidable by-product during the synthesis of epichlorohydrin from allyl chloride. Years of working near pilot-scale chlorination units reveal a process that's deceptively simple: pass chlorine gas through a cooled reactor containing propylene under carefully controlled conditions. This simplicity is offset by the challenge of controlling yields and minimizing co-products. The advent of aqueous-phase catalysis and advanced distillation systems has helped curb emissions and boost selectivity, but the chemistry demands vigilance. Small leaks or spills leave a lingering odor that stays in the air and on clothing longer than most care to remember.
Lab books fill up quickly with experiments using 1,3-dichloropropanol as a substrate. Whether reacting with strong bases to form epoxides or coupling with ammonia sources to introduce nitrogen atoms, its versatility underpins much of organic synthesis. Its greatest power comes from the two chlorine atoms, sitting at each end of a three-carbon chain, acting as anchors for chemical transformations. Over the years, I've run reactions with sodium azide to produce azido-derivatives, and with sodium hydroxide to yield glycidol after hydrolysis. Its value comes from being easy to modify and integrate into larger, more complex molecules without requiring exotic conditions or reagents.
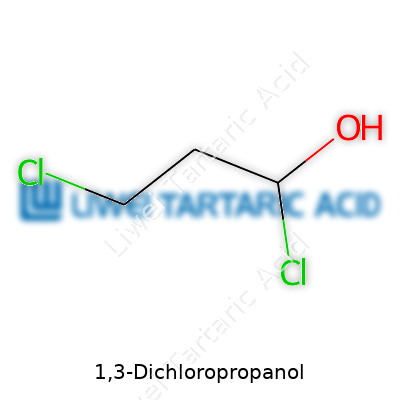
Across catalogs and regulatory documents, 1,3-dichloropropanol masquerades under several names: 1,3-DCP, α,γ-dichloropropanol, and even the less-official “dichloropropyl alcohol.” The string “1,3” indicates the two chlorines bookending the propanol chain, distinguishing it from 2,3-dichloropropanol, which has different uses and risk profiles. Each name reflects a slightly different context—academic, regulatory, or industrial—so recognizing the aliases keeps communication clear when tracing data in studies or batch logs.
Handling 1,3-dichloropropanol requires strict application of chemical hygiene best practices. No shortcut replaces personal protective equipment: goggles, gloves, and well-ventilated hoods. After nearly two decades in analytical labs, I've seen complacency lead to accidental eye or skin exposure, always causing irritation and sometimes triggering more severe symptoms. Beyond personal risk, plant settings adopt engineering controls to limit fugitive emissions, following OSHA exposure guidelines and the ACGIH threshold limit value. Environmental discharge gets monitored continuously, as even small releases can draw attention from regulators and the public. Facilities handling it at scale pursue ISO 14001 or Responsible Care certifications, using rigorous documentation and training to track every gram from arrival to disposal.
1,3-Dichloropropanol’s strongest commercial value appears in the manufacture of specialty chemicals like surfactants, epichlorohydrin, and as a reagent in pharmaceutical synthesis. In my experience consulting for flavor and fragrance companies, industry practitioners often encounter 1,3-dichloropropanol as a trace contaminant rather than a target ingredient. Food safety labs keep an eye out for it due to concerns about carcinogenicity, particularly in products like soy sauce made with certain acid hydrolysis techniques. Manufacturers on a global scale needed to update methods or change ingredients once routine tests began detecting 1,3-DCP at nanogram levels. It’s a reminder that chemistry’s hidden detours can show up where they’re least wanted.
Current research keeps circling back to two key problems: finding greener methods of making epichlorohydrin without generating 1,3-dichloropropanol, and developing sensitive assays to detect trace levels in food and water. Over the past decade, university and government labs explored catalytic pathways using metal oxides or biotransformation using bacteria. I’ve worked with teams monitoring enzyme-catalyzed routes that skip over the DCP intermediate completely, but commercial adoption still faces challenges around cost and process reliability. On the analytical side, real progress came with the refinement of gas chromatography-mass spectrometry methods, shrinking detection limits well below regulatory thresholds. Research funding now targets remediation strategies, aiming to pull DCP from groundwater in contaminated industrial areas.
Studies in toxicology show troubling results for high-dose exposure—even brief contact can irritate skin and eyes in humans, while chronic exposure links to liver and kidney damage in animals. The International Agency for Research on Cancer lists 1,3-dichloropropanol as a possible human carcinogen, which shapes both workplace exposure rules and public food safety campaigns. My role on a chemical risk committee taught the importance of not waiting for new incidents. Synthetic flavor manufacturers started routine checks in the late 1990s, once it became clear that process changes could slice exposure in half with little disruption. Long-term studies keep raising new questions, especially about how DCP accumulates in groundwater or migrates in packaged foods.
The path forward for 1,3-dichloropropanol faces tight scrutiny from environmental groups and government agencies, yet its underlying chemistry still offers promise. Researchers keep searching for bio-based routes that make large-scale production cleaner, while sensor developers chase better ways to spot DCP at minuscule concentrations. My view is that regulatory pressure will only increase, and companies that invest in safer processes or alternatives will stand out both for public trust and long-term profits. Chemical engineers balancing performance and safety now take cues from consumer feedback just as much as new reaction mechanisms, blending practical ingenuity with scientific discipline to keep pace with expectations and obligations.
1,3-Dichloropropanol usually pops up in conversations about chemical manufacturing or environmental risk. This chemical comes from processing during the making of some major solvents, and it’s sometimes formed during the production of epichlorohydrin, which feeds into plastics, resins, and glycerin production. When bigger companies push out products made from those materials, 1,3-Dichloropropanol is often an unintended byproduct. What's more important is what happens to this chemical once it comes into play, because rarely does it end up somewhere positive.
Some people started hearing about 1,3-Dichloropropanol not because of labs or factories, but because this chemical can end up in food. Processing with fats or oils, especially when using chlorinated compounds or tasting the result of certain preservatives, sometimes creates tiny amounts of this chemical. I’ve seen research suggesting that Asian sauces—think soy or oyster sauce—can sometimes carry microgram traces. For anyone who dines out a lot or enjoys groceries from around the world, this kind of information hits home fast.
Groups like the World Health Organization and the European Food Safety Authority flagged 1,3-Dichloropropanol as a “possible human carcinogen,” based on available animal data. That sends a clear signal: even in small amounts, we have reason to be thoughtful about how it enters the food chain. Eating safe food matters for everyone, from toddlers just starting school to older folks managing long-term health.
One place where this chemical shows up more than anyone would want is around industrial sites. Wastewater from plants sometimes carries traces of 1,3-Dichloropropanol, and it only takes a small leak to affect a water source, especially in towns that rely on surface water or shallow wells. Fish in nearby streams do not thrive in chemical-laden water, and local communities have to push hard for regular monitoring and transparency from companies.
Workers face the highest risk. Breathing in vapors or coming in contact during the manufacturing process can lead to symptoms like skin or eye irritation. With enough exposure, research points to higher risks for long-term health problems. Anyone who has worked in an old chemical plant knows that even the best-run safety programs only help if everyone follows the rules—from the plant manager to weekend contractors. This isn’t something that just “takes care of itself.”
Better controls in industrial waste streams stand out as a smart start. High-quality filtration, better chemical tracking, and regular testing for residues should not be out of reach for major companies. Governments can set enforceable limits and watch for violations with advanced, sensitive detectors, which can now spot even tiny parts per billion.
On the food side, manufacturers need incentives to adopt cleaner processing or use chlorine-free chemicals. Popular soy sauce brands in Europe have already begun switching practices after safety alerts went public. Labelling for full transparency, paired with research and education for both manufacturers and consumers, helps everyone make safer choices.
It’s impossible to wrap every ingredient in endless testing. Staying informed, keeping standards high, and speaking up as both a citizen and consumer pays off long-term. There’s no room for shortcuts on health or the future of the environment.
1,3-Dichloropropanol, a mouthful of a name, pops up in food safety talks and chemical plant reports. You find it in the food processing world, usually as a byproduct when fats or oils get heated up with chlorine-containing compounds. That means it sometimes sneaks into everyday stuff, especially certain soy sauces or processed foods. It’s also used as an intermediate in the production of chemicals and sometimes lingers in the water near factories that use it.
The major scientific concern comes from several rat studies. Researchers have flagged 1,3-dichloropropanol for its links to cancer in lab animals, especially around the liver and kidneys. The International Agency for Research on Cancer (IARC) calls it “possibly carcinogenic to humans.” While we don’t have proof straight from human studies, cancer risks found in animals matter a lot in public health. Lab work also confirms that even low levels can cause DNA damage in cells. DNA damage often shows up first in cancer storylines. The European Food Safety Authority stays pretty blunt about it—this chemical doesn’t really seem to have a safe threshold.
It doesn’t just end at cancer. Prolonged or high exposure can mess with your central nervous system, kidneys, and liver. People handling it directly during manufacturing have reported dizziness, nausea, and skin irritation. Ingesting it in food or drink, even tiny amounts over years, whittles away at body defenses, especially for kids and people with chronic health problems.
Laws show the risk gets taken seriously. In the European Union, food products like soy sauces have set legal limits for 1,3-dichloropropanol—20 micrograms per kilogram. Japan also watches its food chain. The US Environmental Protection Agency monitors the chemical, keeping an eye on residues in drinking water and industrial waste. Producers can’t just dump waste that might contain it. Most countries ask companies to run repeated tests, especially for products heading out for export.
Few people actually know what slips into their food from big factories. So, finding out a byproduct like 1,3-dichloropropanol lands on dinner tables feels like a breach of trust. After years working across food companies, I’ve learned that chemical risk isn’t just about “levels” or scary-sounding names. Big factories sometimes avoid using chlorine, choosing other methods, but these processes can cost more. Cheaper, outdated methods let the byproduct build up. Food safety watchdogs usually do a decent job, but smaller companies and manufacturers in countries without strict regulation don’t always follow best practices. That leaves holes in the worldwide food supply chain.
For me, one lesson keeps coming back: more rigorous testing and clearer labels empower buyers to protect themselves. Companies could move toward greener processing steps—ones that don’t generate hazardous leftovers like 1,3-dichloropropanol. Public pressure works—I’ve seen factories overhaul recipes overnight once journalists or consumer groups drew enough attention. On a home level, stretching outside processed foods, cooking basics at home, and choosing brands known for transparency cuts down exposure. Governments and businesses both hold the power to shrink this hazard, but the push often starts with people demanding better answers about what’s in their food.
Most people outside chemistry labs haven’t heard of 1,3-dichloropropanol, but those who handle chemicals at work know it’s nothing to ignore. This clear liquid carries risks linked to skin absorption, inhalation, and even ingestion. A splash on the skin brings irritation, redness, or burns—nobody wants that. If the vapors linger, breathing can get harder, eyes water, and headaches set in. Exposure over time brings bigger trouble, ramping up cancer risks. Regulators in the US and Europe flag it for strict controls because of this.
Standing in front of a fume hood, the gear you choose makes all the difference. Gloves aren’t just a suggestion—they are non-negotiable. Nitrile or neoprene gloves offer a strong barrier. Some folks use latex by habit, but 1,3-dichloropropanol cuts through latex too fast. Goggles fit tight against your face—splashes sneak into eyes in seconds. A chemical-resistant lab coat guards arms and torso, and closed-toe shoes keep accidental spills off your feet. Masks fitted with organic vapor cartridges keep lungs safe because a cotton or dust mask doesn’t help against chemical vapor.
One lesson I learned early on: never underestimate fumes. Flammable liquids like this demand a well-running fume hood. Just opening a bottle in a closed room puts everyone at risk. If the ventilation system hums louder than the rest of the equipment, technicians usually take it as a good sign. In a spill, speed and confidence matter. Alert others, rope off the area, and grab chemical spill kits—never try to mop it up with paper towels like regular messes. Safe cleanup uses absorbent pads made for chemical spills, and all waste goes into properly labeled containers. Teams train for these moments, running through drills repeatedly.
Leaving a bottle of 1,3-dichloropropanol in the wrong spot tempts disaster. Locked chemical cabinets, away from heat sources or open flame, make the right storage solution. You’ll find labels warning about flammability and toxicity; nobody should transfer liquids casually into unmarked bottles. Clear labeling with hazard symbols turns confusion into clarity. Even people just passing through a workspace need to recognize these warnings at a glance.
Most chemical accidents start with a skip in routine. New team members sometimes rush through safety presentations, thinking spills only happen to others. Regular drills, real stories about injuries, and full walkthroughs of safety gear help the warnings stick. Supervisors run spot checks, not to punish but to show that caution saves fingers, lungs, and careers. If you only follow the steps because someone’s watching, one tired afternoon could lead to a lifetime of regret.
Some labs switch to safer alternatives whenever possible, cutting out risky substances like 1,3-dichloropropanol in favor of milder chemicals. Still, this isn’t always possible in production or research. Open communication and easy-to-access Safety Data Sheets give teams the facts needed to protect themselves. As more people raise questions about chemical health, manufacturers and regulators keep a closer watch, while labs double-check their training and equipment. Safer habits build up over time, making labs stronger for everyone who spends their days working inside.
Most people rarely think about chemicals like 1,3-dichloropropanol, yet molecules like this play big roles in industry and sometimes end up in places you wouldn’t expect. The chemical formula for 1,3-dichloropropanol is C3H6Cl2O. That short string of letters and numbers signals a lot about what gets into our environment, food, and even water.
A close look at the formula tells you the molecule carries three carbons, six hydrogens, two chlorines, and one oxygen. This combo points to a member of the halohydrin family: a group that shows up everywhere from food manufacturing to industrial cleaning agents. Having spent time speaking with water engineers and folks in food safety labs, it’s clear that monitoring for trace contaminants isn’t just busywork — it often starts with knowing exactly what sort of chemicals could show up.
In my own experience following the aftermath of accidental spills, I’ve seen how 1,3-dichloropropanol sometimes enters wastewater after industrial mishaps. Since the compound breaks down slowly, it may travel farther than people would guess, raising concern for groundwater.
It’s not just a question of structure. 1,3-dichloropropanol doesn’t sound nearly as alarming as lead or mercury, but over time, exposure adds up. The molecule’s chlorine atoms not only boost its toughness but increase risks for toxicity. Scientists have linked the substance to potential carcinogenic effects, and several regulatory agencies see it as a likely human carcinogen. The chemical can develop as a byproduct in food processing, especially in hydrolyzed vegetable protein production, likely landing in various sauces and seasonings – trace, but not zero.
The layers of food safety have a lot more to do with smart chemistry than marketing would let on. If your day job involves inspecting factories, knowing the difference between 1,2- and 1,3-dichloropropanol isn’t just trivia – it means catching mistakes before they land on a dinner plate. The formula C3H6Cl2O spells out a clear fingerprint. With the right test, it’s possible to catch the molecule in water or food before people are exposed to harmful levels.
Companies face pressure to cut out or reduce byproducts like 1,3-dichloropropanol. Practical solutions come from using alternative production methods, tweaking raw materials, or installing better monitoring systems. Labs today offer sensitive detection tools, including gas chromatography and mass spectrometry, allowing food and water labs to keep tabs on levels down to the part-per-billion range. My conversations with workers in the field point out that real improvements follow from having affordable, quick tests. Public reporting on industrial releases also creates transparency and puts pressure on laggards.
Paying close attention to what chemical formulas really mean – not just for the chemistry but for real-world health – keeps communities safer. That string, C3H6Cl2O, serves as a reminder that even small molecules can draw a line between safe food and unnecessary risk. Gaps in oversight almost always show up first in overlooked sectors. With more focus on prevention, we can shrink the chances for exposure before anyone realizes there’s even a problem.
Having worked in labs where carelessness brought plenty of grief, I’ve learned that storing chemicals isn’t just about following a checklist—people’s safety rides on these choices. 1,3-Dichloropropanol doesn’t belong in a cluttered storeroom or wedged between general-use chemicals. This clear, colorless liquid brings toxicity with it, linked to organ damage and sometimes cancer in lab tests. Folks have gotten seriously sick just from skin exposure or a whiff in the wrong space. If regulations feel like overkill, consider the people who mop the floors or fix broken pipes. Even the best-ventilated facility stands at risk if someone cuts corners.
A locked, ventilated chemical storage cabinet designed for corrosive and toxic liquids works well for 1,3-Dichloropropanol. Keep temperatures cool, and avoid direct sunlight. Heat ramps up vapor pressure, sending toxic fumes into the workspace. The fewer hands with access, the better. Use spill-proof, chemically resistant containers with clear labeling. I once saw an intern mistake a solvent bottle for water—labels matter more than we think.
Incompatibility lurks as well. 1,3-Dichloropropanol doesn’t get along with strong oxidizers. If it leaks or mixes with those, you could end up with a runaway reaction—potential fire, fumes, or worse. Store it on a shelf away from such troublemakers. Simple routines, like regular checks for leaks, keep small problems from turning into disasters. It’s hard to catch a slow drip under a bottle until the day someone gets a rash or the air smells off.
No matter how well you label or shelve, accidents still happen. Gloves made of nitrile or neoprene, splash goggles, and a lab coat give you a fighting chance if something spills. Chemical fume hoods aren’t just for reactions—they’re the best place for any handling. Breathing an irritant like this sets you up for sore throats and worse if you skip the basics.
Flushing toxic waste causes long-term headaches for everyone. Your local water treatment plant can’t magically make nasty chemicals disappear—they end up back in rivers or groundwater, hurting wildlife and endangering drinking water. In my experience, bottles labeled “waste” still wound up in general trash when folks felt pressed for time. This never ends well.
Segregate 1,3-Dichloropropanol waste in sealed containers, away from acids and bases. Mark it clearly—this isn’t just about compliance; it keeps the next shift from nasty shocks. Hazardous waste contractors pick up these materials, depending on local environmental laws. Ask about pickup schedules and treat storage time limits like gospel. When a site tries to save money by holding waste too long, leaks and overflows creep up fast.
Solid training beats fancy equipment every time. A team that knows what a spill kit looks like, who to call, and why double-bagging matters stands safer than one relying on a binder no one opens. Use checklists. Audit your space and track near misses: these stories push management to invest in safer materials or better training. For those working alone, sharing these points saves crises—from lost wages to ruined health. Good chemical hygiene defends not just the workplace, but every community downstream of it.