1,3-Dichloro-2-Propanol has a story that's older than most people imagine, with its roots stretching back to the era when chemical industry expansion started to bring new molecules into common use. Chemists discovered it as they investigated ways to create intermediates for larger, more complicated organic compounds. It gained attention during the mid-1900s, a time when synthetic chemistry rushed to meet the demand for new materials, plastics, and pharmaceuticals. Over decades, its production processes evolved, reflecting growing concerns about workplace safety and environmental impact, but the chemical’s fundamental structure and uses remained largely the same. My recollection from years in labs is that research papers cited it as a basic building block, especially during the chemical boom after World War II, pointing to its importance in fine chemical synthesis.
Placing 1,3-Dichloro-2-Propanol on the bench, it serves as a versatile intermediate, mainly in the manufacture of epichlorohydrin, resins, and various chemical synthesis chains. It plays a role in producing pharmaceuticals and has cropped up in the creation of certain pesticides. Experts often call it a “workhorse intermediate”—not glamorous, but reliable. The chemical enters food and flavor chemistry as well, though concerns about toxic byproducts pushed many industries to rethink or monitor its use. Regulations reflect its double-edged status: useful for industry, problematic for health and the environment.
In daily handling, I remember the sharp, pungent odor that signals its presence from a distance. 1,3-Dichloro-2-Propanol usually presents as a clear or slightly yellow liquid, boasting high miscibility with organic solvents but showing limited solubility in water. Its boiling point sits at approximately 174°C, and it has a melting point near -4°C. The density falls around 1.38 g/cm³, making it heavier than water. Its reactivity with nucleophiles and bases creates opportunities for synthetic routes but also demands respect during handling, as accidental exposure can lead to skin and mucous membrane irritation.
Any bottle in the storeroom there gets a label showing its chemical name, formula (C3H6Cl2O), CAS number (96-23-1), hazard classification, and UN number (UN 2689). Packaging must shield it from light, air exposure, and moisture. From my own practices, every shipment comes with a Safety Data Sheet that outlines PPE, safe handling, spill containment, and firefighting measures. International transport regulations add a layer of detail, listing it as a hazardous substance under GHS guidelines. Handling protocols rarely change across borders, given its classification as a potential carcinogen and environmental hazard.
The most common route to 1,3-Dichloro-2-Propanol involves treating glycerol with hydrochloric acid or by the chlorination of allyl alcohol. These reactions require precise temperature control, acid-resistant equipment, and adequate ventilation—mistakes can introduce byproducts or dangerous fumes. Industrial plants gather thousands of liters in batch reactors, directing waste streams through neutralization towers to cut environmental burden. I recall that research-scale syntheses often take place under fume hoods, with procedures taught and retaught by senior chemists to minimize operator risk. Waste handling plays a significant role, with authorities strict about chlorinated organic disposal due to persistent groundwater pollution.
As a building block, 1,3-Dichloro-2-Propanol undergoes nucleophilic substitution, oxidation, or reduction to yield epichlorohydrin—one of the most important intermediates for epoxy resin production. Its reactivity allows chemists to swap one or both chlorine atoms for other functional groups, creating a web of derivatives. In research, it serves in synthesizing glycidol, alcohols, esters, and heterocycles. The spectrum of reactions is broad, running from classic SN2 mechanisms to less predictable rearrangements at higher temperatures or in the presence of strong bases. Literature points to its use in pilot projects for pharmaceutical ingredient synthesis, especially where a dichloropropanol backbone gives an entry point to more complex molecules.
Depending on language and market, one can run into a host of names: 1,3-DCP, alpha, gamma-dichlorohydrin, Glycerol dichlorohydrin, and Dichlorohydrin among them. International supply chains may also list variants in labeling, such as Dichloropropanol-1,3 or 2-Propanol, 1,3-dichloro-. Packaging inserts or regulatory documents often list synonyms to avoid confusion, especially during customs checks and when interfacing with multinational companies or researchers accustomed to different nomenclatures. Familiarity with synonyms cuts miscommunication, especially in procurement, shipping, or regulatory review.
Direct handling of 1,3-Dichloro-2-Propanol in the lab or factory means donning gloves, goggles, and, for sizable processes, full-face protection and respirators. Its vapor proves irritating, prompting proper fume extraction and closed systems in larger operations. Chronic exposure links to liver and kidney toxicity and possible carcinogenicity—a point hammered home during hazardous materials training. Chemical hygiene protocols call for rapid spill cleanup with appropriate sorbents and ventilation, and routine air monitoring in workplaces. Emergency eyewash and showers need positioning near storage and reaction areas, with drills and signage reinforcing readiness.
Manufacturers use 1,3-Dichloro-2-Propanol mostly as a stepping stone in the synthesis of epichlorohydrin, which in turn forms the foundation of resins used from aerospace to electronics. It also appears in certain biocidal formulations, though regulatory changes in Europe and North America restricted this use over worries about food contamination and water quality. Flavor and fragrance makers historically found it in artificial flavor synthesis—recent food safety rules sparked reformulation and tighter controls. Researchers investigating medicinal chemistry take it into account for constructing specific drug molecules, but the toxic profile of the parent compound limits direct pharmaceutical use.
Scientists continue to study new catalytic systems aiming at greener synthesis routes, with the hope of lower energy consumption and reduced toxic byproducts. I remember meetings where the industry panel pressed for process intensification and routes with higher selectivity. Efforts to find biocatalytic alternatives or recycle raw materials in closed systems receive funding, emphasizing both cost savings and sustainability. Universities collaborate with industry partners to publish findings on milder chlorination approaches, and regulatory science teams monitor levels in consumer products and the environment. Government guidelines, such as those from the European Food Safety Authority, often spark research into analytical detection or risk assessment at lower thresholds.
Controversy surrounds this compound’s presence in foods and the environment. Toxicology studies report links to liver toxicity, genotoxicity, and suspected carcinogenicity in animal studies, leading to strict limits for workplace exposure and maximum allowable concentrations in foodstuffs. Surveys of drinking water in industrial areas picked up trace contamination, leading to calls for better remediation and source controls. Toxicity drives regulatory oversight—public databases like TOXNET and publications in journals press researchers to discover new detoxification technologies. Some labs explore bio-based breakdown of industrial waste containing 1,3-Dichloro-2-Propanol, but with variable success.
Looking ahead, demand for epichlorohydrin, and therefore 1,3-Dichloro-2-Propanol, is expected to remain steady, especially in the composites sector. At the same time, tightening regulations and rising consumer awareness about food and water contamination mean producers and researchers need to innovate. High on the research agenda: new catalysts, more efficient process design, and ways to replace harsh chlorination steps. Labs in Europe and Asia race to patent solutions that could reduce accidental releases and lower persistent contamination. If recent green chemistry initiatives realize their goals, the future could see this legacy intermediate hanging on—albeit in safer, cleaner form.
Many people walk past chemical names like 1,3-Dichloro-2-Propanol without much thought, but this compound leaves a mark in more places than most folks realize. Chemists came up with this molecule for a reason: it acts as a handy building block in several industries. Its uses highlight how chemical ingredients work their way into our lives, sometimes without us knowing.
The story really starts in the chemical factories. 1,3-Dichloro-2-Propanol helps create other chemicals. Most often, companies use it to make epichlorohydrin, an ingredient that eventually becomes epoxy resins. Those resins get shaped into tough coatings, glues, and parts for cars and planes. Take a look at construction, electronics, or even wind turbines, and epoxy resins show up everywhere, often behind the scenes, keeping things together. Without chemicals like 1,3-Dichloro-2-Propanol stepping in at the early stages, many of these modern conveniences just wouldn’t exist.
This chemical isn’t just for factories carving out parts or making paint stick stronger. It can show up in research labs and crop up during the production of certain drugs. Chemical intermediates like this make it possible for other, more useful, or more valuable compounds to come to life. Each step in the lab sometimes calls for small but mighty chemicals like this one.
Not everything about 1,3-Dichloro-2-Propanol is rosy. The same chlorine atoms that make it good at getting chemical work done can also turn it into a health hazard. Studies point to possible cancer risks—so workers handling this chemical need to stay protected with serious safety gear and well-ventilated spaces. The International Agency for Research on Cancer (IARC) labels it as possibly carcinogenic to humans. That’s not a warning any of us can ignore, especially since some countries have tracked its residues in food, particularly in soy sauces from certain regions, likely due to manufacturing shortcuts.
Regulations in Europe and the US have tightened around exposure limits, aiming to keep both workers and the public out of harm’s way. Agencies like OSHA and the EPA track this chemical closely, setting limits for both production and accidental releases. This approach has driven safer workplace practices and sent a clear message: short-term profits can’t come before long-term health.
Chemists have worked on greener or safer alternatives for years. The focus now falls on cleaner processes and tight quality checks. Companies investing in better filtration, real-time monitoring, and research into substitute materials can cut down on the risks. Tougher checks—beyond just paperwork—keep food products safer and make sure that shortcuts don’t slip through the cracks.
Communities living near chemical plants, and consumers feeling wary about what’s in their food, deserve clear, honest information about chemical risks. Open data and accountable regulation can keep workers, neighbors, and customers informed and safe. Technology and training help too. Up-to-date sensors, strong oversight, and a stubborn commitment to safety help prevent accidents before they begin. In my own work, I’ve seen how the smallest leaks can cause headaches for entire teams. Taking chemical safety seriously and investing in simple solutions—like sealed systems and frequent inspections—beat fancy promises every time. That’s why society as a whole keeps an eye on chemicals like 1,3-Dichloro-2-Propanol.
1,3-Dichloro-2-propanol or 1,3-DCP shows up in industrial processes, and scientists keep a close eye on it because of what it can do inside the body. This compound often gets attention in the food world since it can appear as a contaminant in refined vegetable oils and in some processed foods. The first time I learned about it, it was during a conversation about food safety, not in a chemistry class — and that’s telling. Most people come at it through what they eat, not by touching it in a lab.
1,3-DCP has a pretty rough reputation in public health circles. Studies done on animals show it can damage the liver and kidneys, and scientists link it to increased cancer risks. Regulatory agencies in Europe and Asia flagged it after animal tests revealed tumors following repeated exposure. For those working in industrial settings where this stuff is produced or used, inhalation, skin contact, or accidental swallowing could result in poisoning. Even at low levels, if contact goes on for long enough, problems start cropping up.
One story stands out from a health journal, describing factory workers who had above-normal liver enzymes in their bloodwork after years spent around 1,3-DCP. This isn’t some rare event — repeated workplace complaints have kicked off research projects and changed safety guidelines. While workplace exposure remains the main risk for adults, the food angle shouldn’t get ignored. European Food Safety Authority studies found that people, including children, can ingest measurable amounts in common foods. So, even outside factories, levels get high enough to draw attention from regulators.
People sometimes shrug when they hear about another “chemical risk.” But food supply safety isn’t just theory. I’ve seen how recalls and regulatory crackdowns play out — media stories break, grocery shelves sit empty, and families ask honest questions about what lands in their shopping carts. Experts warn that 1,3-DCP stays stable in food processing and rarely breaks down under normal cooking, so keeping it out of the food chain in the first place takes priority.
From a scientific angle, research points to 1,3-DCP forming as a byproduct during oil refining and in the production of some food ingredients. Processing conditions with high temperatures and the presence of certain additives can boost its formation. So, solutions start at the design stage. Food producers adjust manufacturing processes, look for alternative methods, and screen incoming materials. Workers in related industries need tough safety training and good ventilation systems. Gloves and protective gear aren’t optional after seeing the long-term damage done in places without them.
Many countries already set strict limits for 1,3-DCP in foods and enforce tougher rules in workplaces. Agencies like the International Agency for Research on Cancer list it under substances with clear evidence for causing tumors in animals. Gaps still exist, especially in places where weak regulation lets standards slide. Transparency helps. Manufacturers need to keep supplying solid data about their processes, and regulators must run regular spot checks. Scientists are looking into more precise detection so contamination doesn’t fly under the radar.
The story of 1,3-DCP offers another reminder: keeping food and workplaces safe takes effort on all sides, with real health risks for people who miss the warnings or let shortcuts in safety slide.
Anyone working around 1,3-Dichloro-2-Propanol quickly learns how unforgiving it can be. As a clear or slightly yellow liquid with a strong odor, this chemical brings real risks, particularly to lungs, skin, and eyes. My days in a specialty chemicals plant taught me there’s little room for casual attitudes—for 1,3-Dichloro-2-Propanol, mistakes lead to painful burns or serious breathing trouble. Health agencies, from OSHA to the European Chemicals Agency, warn about its toxic power and possible carcinogenic effects. Keeping workers safe means taking storage and handling rules seriously every day.
Storing this chemical never feels trivial. It breaks down if sunlight sneaks in or temperatures swing too much. Direct sunlight speeds degradation and increases risk of leaks or unwanted reactions. I remember our shift manager insisting on shaded, locked cabinets with steady cooling and robust ventilation. General guidelines put the safe temperature at a cool 2 to 8°C. Piling containers on top of each other leads to leaks or broken seals, so we stored drums on solid pallets, away from high-traffic areas. Good labeling isn’t just bureaucracy; it stops confusion and wrong handling later.
Corrosive properties mean choosing polyethylene, glass, or Teflon-lined steel for containers—regular steel gives up over time. Spill kits and eyewash stations sat within arm’s reach, with weekly inspections. Walking into a messy storage area set off alarms in my mind because a single accident can mean downtime or worse, a hospital trip. Many sites set phosphorus-free fire extinguishers nearby, since chlorine-based fires behave unpredictably. Flammable signs aren’t window dressing; they remind everyone to keep sparks and smoking far away.
Transferring 1,3-Dichloro-2-Propanol isn’t a task for an intern. Properly fitted chemical goggles with side shields, thick nitrile gloves, and long-sleeve aprons go on before the container cap comes off. Splash guards and fume hoods keep vapors out of lungs and off skin. In closed systems, leaks get caught fast, while open pouring runs higher risks—one clumsy move means emergency showers, not just a change of shirt. Training drills where workers practice spill response drills pay dividends. It's not just about rules—it's about building instincts.
No one works alone with this chemical—buddy systems save time during emergencies when someone freezes, alarmed or coughing. Safe work calls for checklists, not shortcuts. I saw what happens when someone skips PPE: painful rashes, eye burns, or ambulance rides. Ventilation fans, not just open windows, clear out dangerous vapors, and keeping a respirator handy is ordinary practice, not paranoia.
Serious hazards demand more than routine hazard labels. Site managers invest in chemical monitoring systems that alert teams to vapor leaks well before they reach dangerous levels. Automation helps remove human error—metered pumps beat hand-pouring every single time. Tight access control limits novice exposure and helps keep the workspace predictable. Training programs that include real-life scenarios, not just theory, build habits that stick for a career.
Government agencies keep pushing for lower exposure limits, and for good reason. Research continues into less toxic substitutes, but until those show up at scale, treating every transfer and every drum as a risk keeps everyone healthier. Safety doesn’t just protect workers; it shields families and communities from long-term health impacts. Treating these protocols as day-one priorities makes a world of difference, in both peace of mind and public trust.
Ask anyone who works in food safety or industrial chemistry, and there’s a good chance the chemical 1,3-Dichloro-2-Propanol (1,3-DCP) gets mentioned sooner or later. Most folks outside that circle don’t give it a thought, but it’s got a tricky reputation. I once worked with a food processing team that switched suppliers after a positive test for chlorinated contaminants, and tracing the source taught me more about global chemical rules than any textbook. It’s a compound that deserves a spotlight, since it’s not just another obscure ingredient.
Countries haven’t landed on the same page on how to control this chemical. Take the European Union, for example. The authorities treat 1,3-DCP as a probable carcinogen. This isn’t just stuffy regulation: the International Agency for Research on Cancer added it to their list of possible cancer-causing agents. In the EU, there are strict upper limits on the amount that can appear in food products, especially in items like soy sauce. The EU takes public health seriously, and sets maximum residue levels to keep exposure in check. The United Kingdom and Switzerland mirror those restrictions in their own codes.
Nobody intends for 1,3-DCP to end up in food. It often appears during processing, popping up as a byproduct in the making of certain foods, especially those that use acid-hydrolyzed vegetable proteins. My experience collaborating with international food labs taught me that producers often face costly recalls if tests catch it above legal limits.
Outside Europe, Japan places similar restrictions. They heavily monitor processed soy products, setting the maximum allowable concentration for 1,3-DCP. Certain testing methods used in Japan become industry standards for quality control around the Pacific.
In contrast, the United States focuses on occupational and industrial exposure, classifying 1,3-DCP as a hazardous substance. Federal agencies like OSHA and the EPA step in when the chemical is handled in factories. They flag it under the Clean Air Act as a hazardous air pollutant, keeping an eye on emissions from manufacturing. The FDA, so far, doesn't hold exact limits for it in food, relying on general food safety oversight.
Concerns over 1,3-DCP keep regulators up at night for good reason. Studies link the chemical to cancer in animal tests, and it’s also toxic to the liver and kidneys. For residents in places where soy sauce is a regular staple, any contamination with 1,3-DCP becomes a serious public health concern. During my years analyzing food import reports, I noticed that shipments flagged for this contaminant almost always faced extra scrutiny, and distributors grew cautious about sourcing.
China recognizes the risk as well, updating food production safety guidelines in recent years. They invest in regular testing to keep levels low, which comes after several high-profile recalls over the past decade. It’s a cycle: public pressure, tighter standards, and investment in better processing technology.
Countries show it’s possible to lower these contaminants with the right controls. Companies that switch to enzymatic hydrolysis or modify production conditions routinely knock down the levels of 1,3-DCP in their foods. The most effective batches I ever tested switched to milder processing agents — and contamination levels dropped significantly.
Better monitoring, clear legal maximums, and investment in safer alternatives set the way forward. Some of this progress comes from global cooperation, with labs sharing new screening techniques and governments flagging suspicious imports. In the end, limiting 1,3-DCP calls for active measures across the whole supply chain, from ingredient sourcing to the very last shipping container.
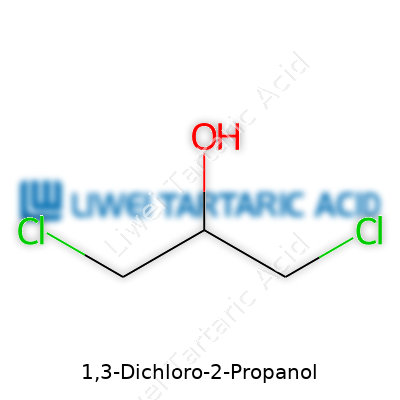
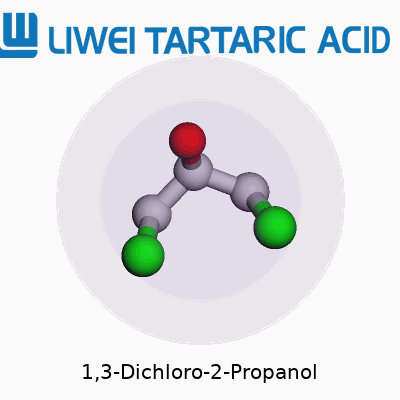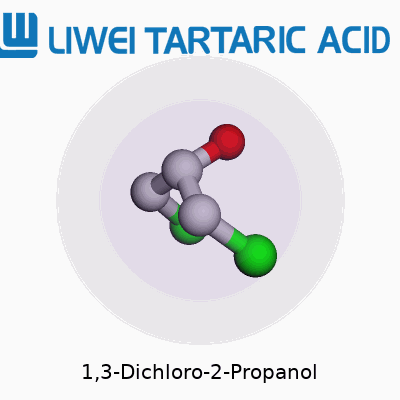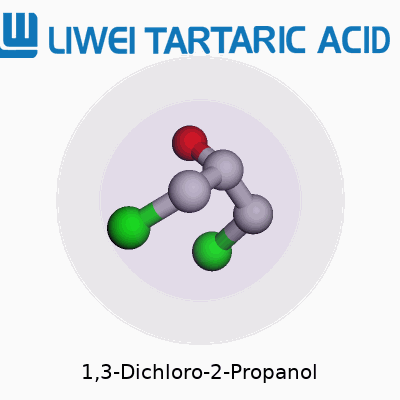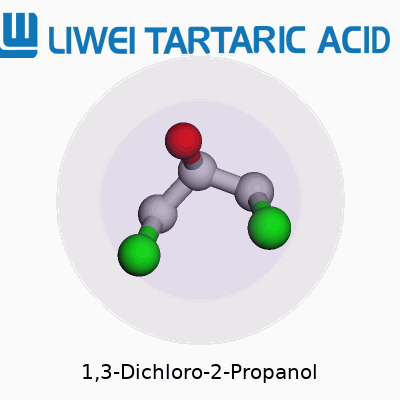
If you’ve ever cracked open an organic chemistry book, you’ll notice some chemicals have a knack for showing up in both lab experiments and industry catalogs. 1,3-Dichloro-2-propanol is one of those. Its structure isn’t just trivia; it plays a role in how people study its behavior and risks. The compound has the formula C3H6Cl2O. That means three carbon atoms linked as a propane chain, decked out with two chlorine atoms and a hydroxyl group. Chlorines anchor at the first and third carbons, and the -OH snags the middle, the second carbon. In skeletal form, you see: Cl–CH2–CH(OH)–CH2–Cl.
Chemists think a lot about these arrangements. The two chlorine atoms don’t just add weight; they change how the molecule behaves and breaks down. You see a direct impact in how the compound dissolves, reacts, and moves through soil or water. There’s value in understanding this at a snooze-level detail, because 1,3-dichloro-2-propanol sometimes appears when larger industrial chemicals break apart—such as in the prep of epichlorohydrin, used in resins and plastics. Sometimes it’s even traced in things as common as processed foods, coming from the breakdown of fats during high-heat treatments. That’s reason enough to dig into the structure and weight, not just for chemical engineers but for public health experts, too.
Each element carries a certain heft. Grab a calculator or even the back of an envelope and add up atomic weights:
Tally these, and 1,3-dichloro-2-propanol lands at about 128.98 g/mol. This number isn’t just academic. In the lab, measuring out doses or figuring out how a pollutant travels starts here. I’ve worked with labs worried about worker safety—molecular weight feeds directly into those calculations. A higher number often means volatility drops, but it takes longer to clear up spills, and the compound grabs onto soil or tissue differently. This matters in cleanup or health guidelines. In the real world, you don’t get to tune out the numbers if you care about exposure risks or product formulation.
People often underestimate how structure links right back to health. That single oxygen wedged between chlorines turns 1,3-dichloro-2-propanol into a chemical worth careful handling. Some research connects it to liver effects or even cancer in animals. It’s not common in daily life, but as labs keep uncovering its presence in industrial waste and food, regulators have set tough restrictions on how much can end up in finished products.
Solutions don’t rest only on what goes into the process; they also depend on what gets monitored after. Investing in better testing and switching to methods that chop down unwanted byproducts has cut down exposure over the years. In my experience, making this shift starts with caring about what each molecule does, not just what’s easiest to measure. Researchers keep trying to design industrial routes that skip these troublesome intermediates. If industry and science stick with it, those numbers—structure and weight—won’t just stay on paper, but translate into cleaner shelves, safer workers, and fewer health questions for people outside the lab.