1,2-Propanediol has been tangled in the fabric of industry since the mid-1800s, with early chemists pulling it from propylene oxide and noting its unique properties. Decades back, the demand for antifreeze brought propylene glycol (another name for 1,2-Propanediol) into the spotlight. The petrochemical boom in the mid-20th century further cemented its place, thanks to efficient synthesis routes developed in major chemical plants. Once seen as a simple glycol, 1,2-Propanediol has shifted from labs to factories and then into formulations stacked on store shelves in everything from cosmetics to plastics.
1,2-Propanediol shows up as a clear, odorless, and practically tasteless liquid, blending easily with water, acetone, and chloroform. Its stable nature and mild scent invited both manufacturers and consumers alike. You see it tagged most often in labels as propylene glycol, PEG, or PG, and the chemical finds its footing in food, pharmaceutical, and industrial segments. Companies rely on its ability to dissolve ingredients and to keep mixtures moist or stable over time.
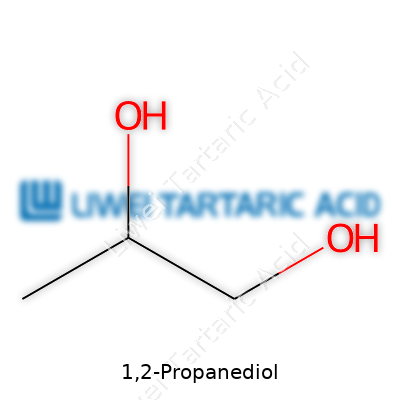
The compound boils up at 188.2°C, which puts it on the higher end for glycols. With a melting point dipping to around -59°C, it stays fluid at almost any room or working temperature. The viscosity sits at about 40 centipoise at 20°C, giving a syrupy but pourable nature. 1,2-Propanediol carries the formula C3H8O2 and a molecular weight clocking in at 76.10. Slight sweetness registers if you take a taste, though that's ill-advised outside food-grade batches. Its miscibility with common solvents and minimal toxicity broaden its range, setting it apart from ethylene glycol, which can poison in small amounts.
Regulations focus on purity, water content, and absence of hazardous contaminants. Food and pharma grades often require 99.5% or higher purity, typical water content under 0.2%, and ultra-low traces of heavy metals or reactive impurities. Batch numbers, manufacturing dates, shelf life, and production origins dominate labeling for transparency and traceability. Companies mark lots for rapid recall or customer assurance, a lesson learned from contamination scares in the last decades.
Industrial synthesis leans heavily on the hydrolysis of propylene oxide. In most plants, the ring-opening of propylene oxide happens either under high-pressure, high-temperature water (non-catalytic route in 200°C territory) or using a catalyst like sulfuric acid. Recent years brought on bio-pathways using glycerol from biodiesel side-streams, slashing carbon footprints. These green methods don't just win environmental points; they stretch profit margins as fossil resources thin out. Each approach comes with trade-offs in energy use, purity, and downstream processing needs.
This glycol's dual hydroxyl groups open doors to a wide palette of chemical changes. Either side gets esterified, etherified, or oxidized depending on the need. Engineers whip up surfactants, plastics, or solvents starting with a simple nucleophilic substitution or dehydration. The backbone becomes building blocks for polyester resins, polyurethane foams, and humectants. Tinkering with the process conditions steers the product toward cosmetic, technical, or food-grade categories.
The market often recognizes this soft, syrupy chemical as propylene glycol. Other trades or patents call it methyl glycol, 1,2-dihydroxypropane, propane-1,2-diol, or α-propyleneglycol. Some older labels read methylethylene glycol, reflecting its roots in historical chemistry books. Brands have also released trademarked blends or specialty cuts fortified with stabilizers, especially for use in pharmaceuticals or vape liquids.
International authorities like the US Food and Drug Administration and European Food Safety Authority have signed off on propylene glycol as generally recognized as safe for food and pharma applications, though a strict watchdog approach governs use levels and labeling. Workers mixing or handling bulk glycol follow local hazard communication standards, proper ventilation, gloves, and eye shields. Storage calls for closed containers, away from heat and oxidants, a lesson learned after a few warehouse fires traced back to outdated storage protocols. As spills rarely pose toxic risks, cleanup still demands diligence to keep floors from turning slick and hazardous.
Propylene glycol forms the backbone of everyday items: toothpaste, lotions, processed foods, pharmaceuticals, antifreeze, airplane de-icers, and hydraulic fluids. As a humectant, 1,2-Propanediol keeps baked goods and tobacco from drying out. Food technologists rely on its ability to dissolve flavors and colors for consistent batch quality. Drug makers harness its stability and safety for oral, topical, and injectable systems, ensuring that actives deliver on their promise. Automotive and HVAC groups keep systems running through winter using blends of water and this safe, recyclable antifreeze. Even e-cigarette liquids depend on this chemical for consistency and delivery.
Researchers push to boost efficiency in bio-based manufacturing. Advances in fermentation and enzymatic processing transform waste glycerol to propanediol, driving a sustainable shift. Start-ups and university labs aim for catalysts that trim by-product formation and energy requirements, focusing on better selectivity and process control. Cross-disciplinary work explores next-generation composites and even medical devices, capitalizing on the compound’s low toxicity and functional versatility. Studies on delivery of drugs, flavors, or actives look to tweak base formulations for climate- and shelf-resistance.
Scientific reviews give 1,2-Propanediol high marks for safety, especially when compared to rival glycols. The US FDA, World Health Organization, and major national health agencies document its metabolic breakdown to lactic acid, a natural constituent in the body. Reports on chronic exposure suggest a low risk, but attention spikes with new delivery modes like vaping. Animal studies show minor irritation in skin or eye contact but very low systemic toxicity unless ingested in large, unrealistic doses. The discussion on long-term inhalation and impact on sensitive populations continues, pushing the science toward even tighter monitoring and reporting.
With the pull toward greener chemistry and renewable resources, 1,2-Propanediol’s fate tracks closely with the success of bio-based synthesis and global regulatory harmonization. Pressure from both environment-friendly consumers and financiers motivates chemical producers to shift from petroleum-based propylene oxide to bio-glycerol routes. The expansion of its use in food, pharmaceuticals, and technical products grows, but regulatory updates follow new application studies—especially for e-liquids and emerging food technologies. As new findings shape regulatory guidance, industry-watchers expect even greater transparency and scrutiny across supply chains. For any company tied to propanediol, adaptability and continuous technical learning mean more than compliance: they form the gateway to innovation and trust in a shifting marketplace.
Open your fridge, grab a tub of ice cream, and you’ll find that 1,2-Propanediol, also called propylene glycol, often plays a quiet role in keeping that treat smooth and creamy. The food industry relies on this compound because it brings stability to flavors, moisture to baked goods, and blends ingredients that typically refuse to mix. A good chunk of packaged and frozen foods sit on shelves longer, and look tastier, thanks to propylene glycol. Food scientists have put it through the wringer over the years, with studies from the FDA and the European Food Safety Authority both backing its safety in modest amounts.
Some folks have voiced concerns about propylene glycol. Too much of it in the bloodstream could spell trouble, especially for those with underlying health conditions or for children. Still, the kind of doses showing up in foods fall well below risky levels. That said, staying curious about what’s landing in our food keeps everyone honest, and encourages transparency in the food industry.
Pharmacists and doctors reach for 1,2-Propanediol for tough jobs: holding together medicines that would otherwise settle or fall apart, especially liquid versions. It acts as a solvent, making active ingredients easier to swallow or inject. One example? Cough syrup. Without propylene glycol, many over-the-counter remedies would separate or lose their punch before the bottle even leaves the pharmacy.
Propylene glycol’s reputation as a friendlier chemical compared to older, harsher solvents helped tip the scales for pharmaceutical companies, eager to avoid toxic or irritating ingredients. Regulators require close scrutiny for additives in drugs, and for good reason—some people have allergic reactions, and rare cases of toxicity pop up when doses climb too high.
Outside kitchens and clinics, propylene glycol brings reliability to machines and buildings. It flows through HVAC systems and industrial chillers, helping regulate temperature without freezing or corroding pipes. Hikers and winter drivers may not realize the de-icing liquid on airplane wings and in car windshields often leans on propylene glycol’s antifreeze properties. Compared to the older ethylene glycol, propylene glycol breaks down into safer byproducts, sparing wildlife much of the ugly fallout if it spills.
Engineers and safety experts keep one eye on environmental impact, though. Propylene glycol doesn’t carry the same reputation for harm as some chemicals, but left to run off in large quantities, it can still tax wastewater treatment systems. Modern facilities set up containment and recycling steps to avoid dumping too much into the water table, but the conversation about greener alternatives never stops.
Experience tells me, most folks would rather not think about what’s keeping their ice cream so creamy or their windshield clear. Still, it pays to look behind the curtain. Propylene glycol shows up in so many corners of modern life, and it’s here because scientists and engineers trust it for reasons rooted in evidence, not advertising. Real transparency about where, why, and how we use chemicals helps everyone—from researchers improving the next best thing, to consumers making informed choices. Sneaking a peek at ingredient lists, asking questions, and holding industries to high safety standards never goes out of style.
Walk through any grocery store and scan the ingredient lists on food, skin creams, or even medicine bottles. Chances are, you'll notice 1,2-Propanediol, more commonly called propylene glycol. On the surface, the name sounds a bit intimidating, but it pops up nearly everywhere. That sort of presence naturally sparks questions among people who care about the things they eat, the lotions they use, and the medicines they trust.
Propylene glycol serves practical roles. It holds moisture, keeps products from drying out, and helps dissolve ingredients that wouldn’t mix otherwise. Think about soft, chewy granola bars or shaving creams that spread easily—many owe their texture and stability to this stuff. In medicine, it acts as a base for drugs that doctors deliver by injection or by mouth.
Looking at a product label as a consumer, my guard goes up with anything that sounds like it belongs more in a lab than a kitchen. So I dig into the research, not just summaries from press releases. Here’s what studies and public health groups say: The U.S. Food and Drug Administration labels propylene glycol as “generally recognized as safe” for its listed uses. Toxicology studies done on animals and people suggest that it doesn’t hang around in the body—it breaks down into normal substances and exits through urine. Reports of allergic skin reactions exist, but these cases are uncommon and usually involve people with sensitive skin.
Europe’s health authorities, Australia’s health department, and the World Health Organization say the same thing: propylene glycol, used within regulatory limits, doesn’t put people at significant risk. The numbers matter here. Most foods and consumer items hold tiny concentrations, far below what scientific models say would spark trouble. Only people with kidney problems or babies under one year face higher risk, since their bodies don’t clear the substance as fast.
No chemical comes without concerns. Some health activists raise points about children, those with allergies, or folks living with kidney or liver disease. I’ve read about rare cases where high amounts of propylene glycol build up from using certain medicines in intensive care. But in daily life, regular doses from food, shampoo, or cough syrup don’t add up to anywhere near these high levels.
That said, more research never hurts—especially since people can have unpredictable responses. I watch for new data or changes in science. Companies should always share clear information and put patient and consumer health first. Regulators regularly review these substances and change their guidance if new hazards pop up.
I talk openly with doctors and pharmacists, especially if anyone in my family has allergies or unusual health problems. Reading labels has become a habit. If a product doesn’t earn my trust or I notice skin reactions, there are often alternatives without propylene glycol. Options matter, and that’s something producers should remember.
Receiving information supports smarter choices. If a product uses propylene glycol, companies ought to explain why and back up their decision with solid scientific reasoning. In my experience, giving consumers clear, honest facts does more for public trust than any long list of chemical names ever could.
Standing in a grocery aisle, scanning ingredient lists, most folks run into unfamiliar terms. Both 1,2-propanediol—also called propylene glycol—and 1,3-propanediol sound like members of the same family. They are. Chemically, each carries three carbons and two alcohol groups. But the way those alcohol groups attach—a difference between the “1,2” and “1,3”—changes how they perform, where they fit in industry, and their safety profiles.
Propylene glycol shows up all the time. It’s found in processed foods, pharmaceuticals, and even in the smoke that clouds a concert stage. It absorbs moisture—helpful for baked goods, tobacco, and pet food. The FDA labels it “generally recognized as safe,” so it sits in products you swallow and put on your skin. I once used a topical gel to treat sunburn, and the key ingredient for its smoothness was propylene glycol.
On the flip side, 1,3-propanediol gives a big boost to the plastics industry. Cosmetic and cleaning products benefit from its gentle touch, especially for people with sensitive skin because it’s less likely to irritate. DuPont first brought it to market for eco-friendlier polyester. You find it in carpets, clothing, packaging, and sometimes in lotions. While propylene glycol’s track record stretches back over a century, 1,3-propanediol’s role as a “greener” plastic ingredient dates back less than 25 years.
The way these two get made sheds light on their benefits and drawbacks. Propylene glycol usually gets synthesized from petroleum, but some newer sources use corn. Its extensive uses rely on fossil fuel-based supply lines, but that’s changing slowly.
People are often surprised to learn 1,3-propanediol can be produced by fermenting plant sugars, like corn or sugarcane. Companies invested millions into this route because of growing concern over oil dependence and carbon footprints. Using renewables appeals to consumers wanting both convenience and sustainability. But the newer production can run more expensive and struggles to supply the giant appetite of manufacturing compared to older, established processes.
Both compounds appear non-toxic—propaganda sometimes clouds this in social media circles. Propylene glycol sometimes gets a bad rap from its distant chemical cousin ethylene glycol, which is dangerous. Study after study supports propylene glycol’s safety, with a few case reports of allergies among sensitive people. Whenever I bake for family gatherings, I pay extra attention when propylene glycol is involved—my cousin reacts with hives.
1,3-Propanediol’s softer skin feel has nudged big brands to give it a try in clean beauty lines. Its carbon savings look good on paper, but lifecycle analyses don’t show a runaway win. More renewable content cuts down on fossil fuel use, yet manufacturing, transportation, and other factors still matter.
Producers already work to blend efficiency with safety, but the push for lower emissions and renewable raw materials seems unstoppable. Better transparency on ingredients and continued independent safety research go a long way. Shoppers can nudge industries by supporting products that clearly label these differences. I’ve learned that paying attention to ingredient lists, and looking up origins, offers more control than most people realize—and nudges companies to keep improving.
Walk down a grocery store aisle or check out the labels on your moisturizer, and propylene glycol (1,2-Propanediol) probably shows up more often than you think. This clear, slightly syrupy substance helps products hold moisture and dissolve other ingredients. It isn’t new to the game—manufacturers have relied on it for decades across the food and personal care industry.
I’ve heard people pronounce anything with a chemical name as toxic, but context matters. The U.S. Food and Drug Administration (FDA) puts 1,2-Propanediol on its list of ingredients that are “Generally Recognized as Safe” when used within certain limits in foods. The European Food Safety Authority also accepts its use, though with strict guidelines on intake. None of these agencies work in isolation; evaluations base themselves in research, repeated testing, and risk assessment.
Stories of propylene glycol hiding in ice cream or salad dressing sometimes raise alarms. Yet, allergies or sensitivities to this ingredient remain pretty rare. Toxicity studies show the body processes small amounts by converting it into lactic acid and pyruvic acid, both harmless when managed by a healthy metabolism. I’ve looked into cases of individuals who needed to avoid this compound, often because of pre-existing allergies or rare metabolic disorders, but the general public rarely runs into trouble at approved levels.
On the skincare side, propylene glycol keeps creams from drying out and helps active ingredients do their job. In my experience, people often react more to fragrances or certain preservatives than to 1,2-Propanediol. Dermatologists recognize it as a low-risk ingredient; high concentrations in industrial applications might cause irritation, but those aren’t present in over-the-counter lotions.
With so much concern around ingredient safety, clear labeling and consumer education can’t get ignored. Knowledge empowers people to make choices that fit their health and values. Open dialogue between consumers, researchers, and regulators fosters trust and helps improve practices. The food and cosmetic industries benefit from ongoing research into both short- and long-term effects. I’ve seen manufacturers responding to demand for transparency by sharing sourcing and purity details, which can go a long way for worried shoppers.
Some suggest alternatives like vegetable-based glycols, but switching always brings its own set of challenges. Reformulating a product changes texture, shelf life, and even cost at scale. I’ve watched small brands experiment with alternatives—sometimes with success, sometimes struggling to match the stability or moisture retention they enjoyed before.
Companies using propylene glycol have an obligation to stay on top of the latest science and adjust their formulas if new hazards appear. Testing batches and adhering to worldwide safety guidelines protect people and maintain trust in the brands. For those with allergies or specific concerns, seeking guidance from healthcare professionals or checking for certified hypoallergenic products offers a practical way forward.
Everybody brings their own values and comfort level to the table. Some folks want food and cosmetics with the fewest additives possible; others value affordability and performance. Having clear, honest information and quality controls means anyone can weigh the risks and benefits before picking what works for them or their loved ones.
1,2-Propanediol, also known as propylene glycol, shows up in lots of different places—industrial coolants, food processing, even cosmetics. At first glance, it looks kind of harmless since you’ll find it in antifreeze and in bottles of moisturizer, but that can lead folks to overlook the basics of safe storage and handling. This isn’t the type of chemical that explodes with a tiny spark, but it still asks for a careful approach.
You can leave propylene glycol in regular warehouse conditions, as long as dampness stays outside the door. In my own experience working in warehouses, humidity sneaks up in corners where containers get stacked too close, or ventilation isn’t up to scratch. Moisture mixing inside a drum can degrade the product or encourage mold growth—nobody wants that. Keeping the product indoors and away from water sources helps it stay fit for use.
Seal the containers tight whenever you’re done pouring or checking inventory. Propylene glycol loves to attract water from the air. Over time, you lose product quality, and water in the solution may cause clumping or change how it flows. I’ve learned that simple actions, like closing lids immediately and labeling drums with the date received, make all the difference for both efficiency and safety.
Lots of warehouses store chemicals side by side, but mixing up propylene glycol with other glycols or with food-grade ingredients can cause big headaches. Proper labeling shows what’s inside and who it’s meant for. I’ve seen storage managers use clear, color-coded tags to make sure the right batch goes out the door. This helps everyone track the shelf life and limits the risk of shipping the wrong material.
Accidents happen, even with the least dangerous chemicals. If a drum leaks or falls, the glycol gets slippery fast. Slippery floors are a hazard. Fitting the storage area with spill kits, absorbent pads, and non-slip mats pays off by reducing injury risk. For hands-on work, gloves and eye protection keep skin irritation at bay. In my own work, gloves and splash goggles weren’t just for the heavy stuff, but for anything that could go sideways in a busy shift.
Propylene glycol isn’t much of a firestarter by itself. Still, storing it near open flames or oxidizing chemicals bumps up the risk. Keep oxidizers in a separate section. Fire codes often call this out, and I’ve seen insurance inspectors double-check storage layouts for this exact problem.
Keeping fresh air moving through storage rooms limits buildup of vapors, especially where drums get opened often. Clean-up routines—wiping drips, clearing up spilled material, washing sticky containers—make the place safer and show respect for the product and the team handling it.
No rule or best practice matters much if the team skips training or skips inspections. Reviewing chemical handling basics during onboarding—and running quick refreshers for everyone—keeps standards high. Routine walkthroughs to spot leaky bungs, faded labels, or messy storage areas can solve problems before they become hazards.
Getting the basics right—dry, cool storage; proper labels; tight seals; strong PPE—and checking in with the team goes further for long-term safety than flashy technology ever could. With food, pharma, and industrial operations depending on pure, stable propylene glycol, attention to detail pays off across the production chain.